RD-58: Thyroid Screening for Radioiodine
Preface
Sections 4(a), 5(2)(a), and 13(1) of the Canadian Nuclear Safety Commission's (CNSC's) Radiation Protection Regulations, (SOR 2000/203) require licensees to implement a radiation protection program that keeps the amount of exposure to radon progeny and the effective dose and equivalent dose to persons as low as is reasonably achievable (ALARA). As part of the program, the licensee is obligated to directly measure and monitor the exposure to radon progeny, and adhere to prescribed effective dose limits for nuclear energy workers (NEWs), pregnant NEWs, and non-NEWs.
This regulatory document provides guidance to licensees regarding recommended elements of a thyroid screening program for workers handling volatile radioiodines, which may be required by licence conditions. Further to the licence conditions, this document includes recommendations for participation in the screening program, instrument selection, the screening method, monitoring periods, and validation procedures.
This regulatory document supersedes R-58, Bioassay Requirements for Iodine-125 and Iodine-131 in Medical, Teaching and Research Institutions, published in September 1983. The focus of this document is on Iodine-125 (I-125) and Iodine-131 (I-131); however, the approach may also be applied to other volatile radioiodines.
This regulatory document is intended to provide guidance for the CNSC's Radiation Protection Regulations (SOR 2000/203), and is partly based on the American National Standards Institute document entitled Design of Internal Dosimetry Programs.
Nothing contained in this document is to be construed as relieving any licensee from pertinent requirements. It is the licensee's responsibility to identify and comply with all applicable regulations and licence conditions.
Table of Contents
- 1.0 Purpose
- 2.0 Scope
- 3.0 Relevant Regulations
- 4.0 National and International Standards
- 5.0 Background
- 6.0 Screening Participation
- 7.0 Instrument Selection
- 8.0 Screening Method
- 9.0 Monitoring Period
- 10.0 Validation of Screening Results
- 11.0 Screening Log
- Glossary
- References
- Additional Information
- Appendix A Volatile Radioiodine Compounds
- Appendix B Instrument Calibration
- Appendix C Sample Thyroid Screening Log for Iodine-131
- Appendix D Quality Control Charts
1.0 Purpose
The purpose of this regulatory document is to provide guidance to licensees with respect to developing a thyroid screening program for volatile radioiodines.
2.0 Scope
This regulatory document describes the recommended elements of an effective thyroid screening program for volatile radioiodines. Thyroid screening of workers handling volatile radioiodines may be required by licence conditions. Further to the licence conditions, this document includes recommendations for selecting participants in the screening program, instrument selection, the screening method, monitoring periods, interpretation of results, validation procedures, and record keeping.
This document supersedes CNSC regulatory document R-58, Bioassay Requirements for Iodine-125 and Iodine-131 in Medical, Teaching and Research Institutions, published in September, 1983. The focus of this document is on Iodine-125 (I-125) and Iodine-131 (I-131); however, the approach may also be applied to other volatile radioiodines.
This document does not apply to the occupational health and safety of workers. The licensee should refer to applicable federal and provincial laws and regulations (see for example the Canada Labour Code ( R.S., 1985, c. L-2 ) and the Ontario Occupational Health and Safety Act, R.S.O. 1990, CHAPTER O.1).
3.0 Relevant Regulations
1. Paragraph 4(a) of the Radiation Protection Regulations states that, “Every licensee shall implement a radiation protection program and shall, as part of that program, keep the amount of exposure to radon progeny and the effective dose and equivalent dose received by and committed to persons as low as is reasonably achievable, social and economic factors being taken into account, through the implementation of iii) control of occupational and public exposure to radiation, ”;
2. Paragraph 5(2)(a) of the Radiation Protection Regulations states that, “A licensee shall ascertain the magnitude of exposure to radon progeny and the effective dose and equivalent dose by direct measurement as a result of monitoring, ”; and
3. Subsection 13(1) of the Radiation Protection Regulations describes the effective dose limits for nuclear energy workers (NEWs), pregnant NEWs, and non-NEWs.
4.0 National and International Standards
This regulatory document is consistent with the philosophy and technical content of modern international codes and standards.
In particular, this regulatory document is partly based on the American National Standards Institute (ANSI) document entitled Design of Internal Dosimetry Programs[3].
5.0 Background
Workers may be exposed to radionuclides in a variety of chemical forms that can be inhaled, ingested, or absorbed through intact skin or open wounds. Radiation protection programs include work area monitoring, such as surface contamination monitoring. Where intakes of radionuclides are possible, radiation protection programs should also include measurements to estimate the quantity of radioactivity deposited in the body or to establish a basis for judgment that significant intakes (in relation to applicable dose limits) of radionuclides have not occurred.
CNSC regulatory guide G-91, Ascertaining and Recording Radiation Doses to Individuals[2], provides guidance to licensees on approaches that may be used to ascertain and record radiation exposures and doses to workers. It includes guidance for ascertaining dose when one or more contributing component of a worker's overall effective dose is likely to exceed 1 millisievert (mSv) per year.
When workers are not likely to receive annual doses exceeding 1 mSv, increased resources for ascertaining dose may not always be justified. Hence other means of assessing possible exposure, such as screening, should be considered.
The purpose of a thyroid screening program is to monitor the intake of volatile radioiodines. Timely information produced by the program is used to assess any intake of volatile radioiodines, provide assurance that the radiation protection program is working, and demonstrate compliance with regulatory dose limits.
In the thyroid screening program, workers submit to an in-vivo count of the thyroid. Results are compared to a predetermined level without the need for dose assessment or intake estimation. Exceeding the predetermined level requires confirmation of intake and an investigation. When a worker frequently exceeds the predetermined level, that worker's need to participate in a routine bioassay program should be re-evaluated.
Although this regulatory document specifically addresses I-125 and I-131, the methods presented in this regulatory document may be applied when developing a screening program specific to other volatile radioiodines (e.g., I-123 or I-124).
Data on I-125 and I-131 is shown in Table 1.
|
Characteristics |
I-125 |
I-131 |
|---|---|---|
Radioactive half-life (days) |
60.1 |
8.04 |
Effective half-life in the thyroid (days) |
40.0 |
7.3 |
Time to maximum thyroid burden after acute exposure (days) |
1.8 |
1.2 |
Main photon energy (kiloelectron volt (keV)) |
27.0 |
364.5 |
6.0 Screening Participation
6.1 Workers - Normal Handling
Workers (NEWs and non-NEWs) who handle a total quantity of open-source volatile radioiodine in a 24-hour period that exceeds the amounts indicated in Table 2, should be screened for I-125, I-131, or both if necessary.
|
Confinement |
Quantity of I-125 or I-131 (in megabecquerel (MBq)) |
|---|---|
Gases, volatile liquids and powders |
|
None |
2 |
Fumehood |
200 |
Glovebox |
20,000 |
Workers should also be screened when they handle other amounts or types of open-source volatile radioiodine in ways other than those listed in Table 2. Appendix A to this document provides examples of volatile radioiodine compounds and illustrates actions that may generate such compounds.
6.2 Other Persons
Other persons who regularly work close to a worker handling more than 2 MBq of volatile I-125 or I-131 on an open bench or in an open area should be screened for the relevant radioiodine.
6.3 Exposure to Spills or Contamination
Workers and other persons who have been exposed to one of the following situations should be screened for the relevant radioiodine:
- Exposed to a volatile I-125 or I-131 spill greater than 2 MBq;
- Externally contaminated by I-125 or I-131; or
- Worked within two meters of a person whose screening measurement results are equal to or greater than 1 kBq, if they were working within one hour after the time of the suspected exposure.
7.0 Instrument Selection
The scintillation detector is currently the most common type of instrument used for measuring radioiodine in the thyroid. It typically consists of a probe (usually containing a sodium iodide (NaI) crystal) operated in conjunction with a counter, a scalar, and a rate meter, a channel analyzer, or a spectrum analyzer. Systems can be as simple as a portable unit that produces results in counts per unit time, or as sophisticated as a gamma spectroscopy system that generates the energy spectrum of the isotope and then quantifies the total activity.
When choosing an instrument, it is advisable to read the instrument specifications carefully or consult with the manufacturer to ensure that the probe and detector are capable of detecting the applicable radioiodine. For more information on selecting a detector for I-125 or I-131, consult the Canadian Medical Radiation Technology publication, Thyroid Monitoring Part VI: Choosing a Detector for Either I-125 and/or I-131.
The NaI detector comes in various sizes and configurations depending on the desired use and sensitivity. When choosing a NaI detector, licensees should consider:
- The gamma energy of the radioiodine isotope to be measured;
- The thickness of the NaI crystal;
- The diameter of the NaI crystal; and
- The window material and configuration of the probe.
7.1 Crystal Thickness
The thickness of the NaI crystal required varies depending on the isotope of radioiodine being measured.
Detection and measurement of I-125 requires only a thin crystal to efficiently detect low energy I-125 photons. Typically, NaI crystals approximately 1 mm thick are used to measure low-energy photon emitters such as I-125.
Conversely, a thicker crystal is required for the efficient detection of higher energy I-131 photons. A crystal approximately 25 mm thick is recommended to detect I-131.
7.2 Crystal Diameter
Another factor to consider is the diameter of the crystal. A large diameter results in greater overall counting efficiency. It also helps reduce error that may result from any variances such as neck-to-detector distances, misalignment of detector with thyroid, and size of thyroid. However, a larger detector diameter increases the background reading.
7.3 Window Material of Probe
The window material of the probe is also a factor to be considered. The low energy I-125 photons require a window material, such as Mylar or beryllium, thin enough to allow the I-125 photons to penetrate the crystal.
If a probe is required to detect both I-125 and I-131, the crystal should be thick enough to detect I-131 and the window material thin enough to allow penetration of I-125.
Table 3 summarizes the recommended specifications for detector uses to measure I-125 and I-131.
|
Specification |
I-125 |
I-131 |
|---|---|---|
Minimum crystal thickness (mm) |
1 |
25 |
Minimum crystal diameter (mm) |
25 |
25 |
Typical window material of probe |
Mylar or beryllium |
Aluminum or stainless steel |
Typical energy range of detector (keV) |
20 - 200 |
8.0 Screening Method
8.1 Set-Up
To install a counting system:
- Set up the counting system in an area of low or at least non-variable background radiation;
- Ensure that the equipment is set up according to the manufacturer's specifications;
- Determine the background count rate using a neck phantom (ideally containing potassium);
- Calibrate the system by following the procedure provided in Appendix B;
- Calculate and record the count rate equivalent to 1 kBq and 10 kBq in the screening log (see Appendix C for a sample screening log); and
- Verify the set-up every time the system or location is changed to confirm its adequacy or make appropriate modifications.
8.2 Quality Control Verifications
To verify the ambient background and the reproducibility of the system's count rate, the following steps should be taken on each day that thyroid screening is conducted:
- Measure and record the background count rate, accumulating at least 400 counts;
- Measure and record the net count rate of a standard source; and
- Record the background and standard source count rates so that deviations from the norm can be readily observed (see Appendix D for a method of recording count rates using control charts).
As a best practice, verify controls annually by participating in a thyroid intercomparison program, such as the one provided by Health Canada and described in the Human Monitoring Laboratory technical report entitled, The Thyroid Intercomparison Program[1].
8.3 Screening Measurement
To perform a screening measurement:
-
Measure the person's background count rate.
This may be done by taking the measurement on the person's lower thigh. Although a thigh measurement simulates iodine that might be in the circulatory system, it should be noted that some detection equipment does not allow thigh measurements. Also, thigh measurements may be affected by contamination of clothes or skin. An alternate method of measuring the background is to use a neck phantom (ideally containing potassium). If the background measurement is higher than usual, verify for possible contamination and repeat the measurement if necessary; - Record the reading in the screening log;
- Measure the person's count rate resulting from the thyroid;
- Record the reading in the screening log;
- Compare the result to the Investigation and Reporting Levels recorded on the screening log; and
- Depending on the measurement results, take the appropriate action based on the options provided in section 10.0 and complete the screening log.
9.0 Monitoring Period
Thyroid screening for I-125 and I-131 on workers and other persons who meet the screening participation guidelines (see section 6.0) should be carried out between one and five days following the exposure.
10.0 Validation of Screening Results
10.1 General Level - Measurement Results ≥ 1 kBq
For all screening measurement results equal to or greater than 1 kBq, the licensee should:
- Verify that the method of screening measurement described in Section 8.3 has been followed;
- If necessary, make any corrections and repeat the measurement;
- If the measurement result is still equal to or greater than 1 kBq, verify clothes or skin for contamination;
- If clothes or skin are contaminated, remove the clothes or decontaminate the skin and repeat the measurement;
- If the measurement result is still equal to or greater than 1 kBq, follow the steps in subsections 10.2 or 10.3, as applicable; and
- Screen all persons who worked in proximity to the person whose results are equal to or greater than 1 kBq.
10.2 Investigation Level - Measurement Results ≥ 1 kBq and < 10 kBq
For all screening measurement results equal to or greater than 1 kBq and less than 10 kBq, the licensee should:
- Validate the results as per section 10.1;
- Perform an internal investigation to determine and correct the cause of the screening results;
- Record the findings;
- Correct any deficiencies found by the investigation; and
- Record the event in the annual compliance report.
10.3 Reporting Level - Measurement Results ≥ 10 kBq
For I-125 and I-131, a 10 kBq result is approximately equal to a dose of 1mSv. Under Section 16 of the Radiation Protection Regulations, the CNSC must be notified when a licensee becomes aware that a dose to a person may have exceeded an applicable dose limit (e.g., 1 mSv per year for a non-NEW). For NEWs, the steps listed below are recommended for consistency with the guidelines presented in CNSC regulatory guide G-91, Ascertaining and Recording Radiation Doses to Individuals[2] . In particular, G-91 recommends ascertaining the effective dose from each component of the dose that contributes more than 1 mSv per year.
For all screening measurement results equal to or greater than 10 kBq, the licensee should:
- Validate the results as per section 10.1;
- Immediately inform the CNSC if the measurement was made on a person other than a NEW;
- Have a radioiodine bioassay performed by a person licensed by the CNSC-if a licensed person is unavailable, the licensee should seek CNSC approval to permit someone else to perform the radioiodine bioassay;
- Use the results of the bioassay to ascertain the committed effective dose;
- Perform an internal investigation aimed at determining and correcting the cause of the screening results, including area and contamination monitoring at the site of the radioiodine intake, if applicable; and
- Record the event in the annual compliance report.
11.0 Screening Log
The licensee should maintain a thyroid screening log. An example is provided in Appendix C.
Glossary
Becquerel
The SI unit for the amount of a nuclear substance.
Effective dose (E)
The sum of the products, in sievert, obtained by multiplying the equivalent dose of radiation received by and
committed to each organ or tissue set out in column 1 of an item of Schedule 1 (Radiation Protection Regulations) by the tissue weighting factor set out in column 2 (Radiation Protection Regulations) of that item.
Effective half-life
The time required for a radionuclide deposited in the body to decrease to one-half of its initial quantity as a
result of the combined action of radioactive decay and biological elimination.
Equivalent dose (HT)
The product, in sievert, obtained by multiplying the absorbed dose of radiation of the type set out in column 1 of
an item of Schedule 2 (Radiation Protection Regulations) by the radiation weighting factor set out in
column 2 (Radiation Protection Regulations) of that item.
Intake
The amount of radioactive material that enters the body through the nose, mouth, skin absorption, or a wound.
NEW
A person who is required, in the course of the person's business or occupation in connection with a nuclear
substance or nuclear facility, to perform duties in such circumstances that there is a reasonable probability that
the person may receive a dose of radiation that is greater than the prescribed limit for the general public.
Other persons
Those who are present at the facility where the licensed activity is carried out but who do not perform work
referred to in the licence.
Radioiodine
A substance containing radioactive iodine in a chemical form which has a metabolic pathway similar to iodide, such
as inorganic compounds and metabolic forms of organic iodine that are broken down in vivo; this includes
the radionuclides Iodine-125 and Iodine-131.
Radioiodine bioassay
The measurement of the amount of radioiodine in the body for the purpose of ascertaining the associated committed
effective dose.
Screening
The monitoring of workers for the purpose of detecting the presence of radioiodine deposited in the thyroid as an
indication of radioiodine intake and is not intended for the purpose of quantitative dose assessment.
Sievert
Unit of equivalent dose, effective dose, and commmitted effective dose (CED). One sievert is defined as one joule of
energy absorbed per kilogram of tissue multiplied by an appropriate, dimensionless, weighting factor. See also
“equivalent dose” and “effective dose”.
Standard source
A radioactive source characterized for the activity of radionuclides by the National Research Council of Canada, or
another national standardizing laboratory for radioactivity measurements, and issued with a certificate that gives
the results of the characterization.
Thyroid burden
The quantity of a radionuclide that has been deposited in the thyroid.
Volatile
Describes a substance that may evaporate at normal temperatures and pressures.
Worker
A worker is a person who performs work that is referred to in a licence.
References
1. Burns, L. C., et al., The Thyroid Intercomparison Program, Human Monitoring Laboratory Technical Report, HMLtd-88-3, Health Canada, 1996, Ottawa.
2. Canadian Nuclear Safety Commission, Ascertaining and Recording Radiation Doses to Individuals, Regulatory guide G-91-2003, Ottawa.
3. Health Physics Society, American National Standard-Design of Internal Dosimetry Programs, ANSI/HPS N13.39-2001, McLean, Virginia.
4. Kramer, G. H., and Meyerhof, D. P., The Canadian National Calibration Reference Centre for In-Vivo Monitoring: Thyroid Monitoring Part III: A Basic Calibration Procedure for Thyroid Monitoring, Canadian Journal of Medical Radiation Technology 25, 2 (1994): 61-63, Ottawa.
Additional Information
The following documents contain additional information that may be of interest to persons involved in designing and implementing a thyroid screening program for radioiodines:
1. Becker, D. V., et al., Society of Nuclear Medicine Procedure Guideline for Thyroid Uptake Measurement, Society of Nuclear Medicine, 1999, Reston, Virginia.
2. Health Physics Society, Performance Criteria for Radiobioassay, N13.30-1996, McLean, Virginia.
3. International Commission on Radiological Protection (ICRP), Radionuclide Transformations, Energy and Intensity of Emissions, ICRP Publication 38, Annals of the ICRP (1983): 11-13, Oxford, UK.
4. Kramer, G. H., and Yiu, S., Thyroid Monitoring Part VI: Choosing a Detector for Either I-125 and/or I-131. Canadian Journal of Medical Radiation Technology 27, 2, (1996): 74-79, Ottawa.
5. Kramer, G. H., and Yiu, S., Examination of the Effect of Counting Geometry on I-125 Monitoring using MCNP, Health Physics Journal 72, 3 (1997): 465-470, Hagerstown, Maryland.
6. National Radiological Protection Board, LUDEP 2.0 -Personal Computer Program for Calculating Internal Doses Using the ICRP Publication 66 Respiratory Tract Model, NRPB-SR287-2000, Chilton, UK.
Appendix A Volatile Radioiodine Compounds
Volatile radioiodine compounds include such compounds as sodium iodide (NaI) and radioiodines in a disassociated form. The volatility of radioiodine compounds may increase as a result of acidifying or freezing.
Activities that may cause radioiodine to be released include opening stock reagent containers, opening packages containing capsules used for therapeutic or diagnostic purposes, and working with such open packages. Hence, capsules given to patients for diagnostic tests or for therapeutic purposes are considered to contain radioiodine in volatile radioiodine form.
The addition of antioxidants, e.g., sodium thiosulfate, to either labelled or NaI solutions reduce both decomposition and volatility. Also, maintaining radioiodine solutions at dilute concentrations minimizes radiolytic decomposition. It should also be noted that radioimmunoassay (RIA) kits contain small quantities of I-125 in a non-volatile form.
Appendix B Instrument Calibration
B.1 General
All equipment and instruments used for thyroid screening should be maintained in good operating condition and calibrated before use. Calibration should be performed for the isotope of interest under conditions mimicking the thyroid in the neck. An example of a calibration procedure can be found in Thyroid Monitoring Calibration Part III: A Basic Procedure for Thyroid Monitoring [4]. Once set up (as per section 8.1) the measurement equipment should be calibrated if a quality control measurement is outside the control limits, as described in Appendix C. Also, the measurement equipment should be re-calibrated prior to being put back into service if it has undergone any significant changes that may have an adverse impact on the precision, accuracy, or reliability of the measurements, such as if the equipment has been repaired or replaced.
B.2 Detector Efficiency
To determine detector efficiency, measure the activity of a traceable standard source of the radioisotope of interest and use the following formula:
E = (C - B)/A
where
E is the efficiency in counts per second (cps) per becquerel (Bq);
C is the measured counts per unit time of the standard source, in cps;
B is the background count rate, in cps; and
A is the known activity of the standard source, traceable to a national standardizing laboratory within 5% (1σ, or standard deviation) accuracy, in Bq.
The licensee should ensure that if an I-131 or I-125 standard source is used, the activity of the source is corrected for decay to the day on which the calibration is performed.
The instrument and measurement parameters used to determine counting efficiency must be the same as those used for routine screening. Placing the detector as close to the thyroid as possible achieves the greatest efficiency. However, due to variations in thyroid depth, size, shape, and positioning, large uncertainties can be introduced into the activity estimate. Such an error can be reduced by increasing the distance between the neck and the detector. Each system has its own optimal compromise between high efficiency and error reduction, but generally a good neck-to-detector distance is 12 centimetres.
The licensee should ensure a long enough count time for both the standard source and background count rate so that overall error (1σ) in the count is less than 5%. This means approximately 400 gross counts for the background measurement.
B.3 Minimum Detectable Activity
The minimum detectable activity (MDA) is the smallest amount of radioactivity that can be detected with a 95% confidence limit. The licensee should conduct measurements so as to achieve an MDA value that is 1 kBq or less. The following formula is used to calculate an MDA value:
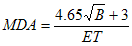
where
B is the total background counts collected during time “T”;
E is the efficiency in cps/Bq; and
T is the time in seconds.
B.3.1 Sample MDA Calculation
If a system's MDA is not low enough-either because of a low efficiency or high background-it can be improved. This can be done by increasing the counting time used for the measurement, or decreasing the background, or both.
As an example, consider I-125. Assuming that the efficiency is 0.0060 cps/Bq and the gross background count is 400 counts, a 300 second count time would result in the following MDA, using the equation in section B.3:
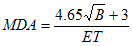
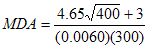
MDA = 53 Bq
The resulting value, 53 Bq, is acceptable for I-125.
The counting time used to monitor personnel does not have to be as long as that used for calibration. In the example above, the background is 400 counts/300 seconds, or 1.3 cps. Assuming the background count rate is stable, a 60 second count time would result in approximately 80 counts. Substituting these new figures into the equation produces a new MDA value, as shown in the following formula:
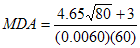
MDA = 124 Bq
B.4 Records
Accurate calibration and service records should be maintained on the measurement system. The records should indicate the following information:
- Calibration date;
- Reference method;
- Calibration source current activity;
- Background values (these should be compared to previous values to detect changes);
- MDA;
- Calculated efficiency; and
- Authorization signature.
Appendix C Sample Thyroid Screening Log for Iodine-131
An example of a Thyroid Screening Log for Iodine-131 is provided in Table C.1.
Table C.1: Sample Thyroid Screening Log for Iodine-131
Investigation Level: ________________________ net cps = 1 kBq of I-131
Reporting Level: >________________________ net cps = 10 kBq of I-131
Employee name: _______________________________________________
Instrument used: _______________________________________________
Date of measurement |
Last use of radioiodine |
Background count rate (cps) |
Gross counts |
Count time (seconds) |
Net count rate |
Technician (Initials) |
Action Taken |
|---|---|---|---|---|---|---|---|
Appendix D Quality Control Charts
D.1 Introduction
This appendix provides a method for recording background and standard source count rates for quality control verifications.
D.2 Control Charts
Prepare two control charts, one for each of the background and standard source quality control verifications described in subsection 8.2. Each control chart should show the date measurements were taken and the corresponding count rates. After approximately 20 days of counting operation, there will be enough observations to estimate the standard deviations for the distributions. The standard deviation can be estimated in the following equation:
S = [(1/n-1)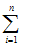 (Ni -
(Ni -
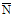 )2]½
)2]½
where
S is the standard deviation;
n is the number of either background or standard source measurements;
Ni is the count rate of each individual measurement; and
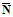 is the average of n measurements of Ni.
is the average of n measurements of Ni.
The licensee should draw control limits at ±2S on each chart. Nearly all the quality control measurements (95%) should lie within the control limits.
If a quality control measurement of the background or standard source is outside the control limits, repeat the measurement immediately. If the repeated measurement is also outside the limits, verify the instrument settings. Finally, if no cause can be found, the licensee may need to take remedial action, including recalibrating or repairing the instruments to ensure subsequent quality control measurements are within the control limits.
Page details
- Date modified: