Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2019
Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2019 (PDF)
Table of contents
- Executive Summary
- Part I: Use of Nuclear Substances in Canada: 2019
- Part II: Class IB Accelerators in Canada: 2018-2019
- Appendix A: Regulatory Program for the Use of Nuclear Substances
- Appendix B: Compliance Performance
- Appendix C: Enforcement Actions Issued in 2019
- Appendix D: Doses to Workers
- Appendix E: Reported Events
- Appendix F: Inspections conducted in 2019
- Appendix G: Regulatory Program for Class IB Accelerator Facilities
- Appendix H: Safety and Control Rating for Class IB Accelerator Facilities
- Appendix I: Effective Doses to the Public from Class IB Accelerator Facilities
- Appendix J: Lost Time-Injury Rate for Class IB Accelerator Facilities
- Appendix K: Effective Doses to Workers at Class IB Accelerator Facilities
- Appendix L: Events at Class IB Accelerator Facilities
- Appendix M: Compliance Rating Level
- Appendix N: Regulatory Documents
- Appendix O: Relevant Regulatory References
- Appendix P: Categorization of Sealed Sources
Executive Summary
This document presents the Regulatory Oversight Reports (ROR) for the Directorate of Nuclear Substance Regulation (DNSR) for the activities falling under its regulatory responsibilities. This report also covers Waste Nuclear Substance licenses, regulated by the Directorate of Nuclear Cycle and Facilities Regulation. The report is provided in two parts:
- Part I: Use of Nuclear Substances in Canada: 2019
- Part II: Class IB Accelerators in Canada: 2018-2019
The Canadian Nuclear Safety Commission (CNSC) regulates the use of nuclear energy and materials to protect health, safety, security and the environment, implements Canada’s international commitments on the peaceful use of nuclear energy, and disseminates objective scientific, technical and regulatory information to the public. Licensees are responsible for operating their facilities safely and are required to implement programs that make adequate provision for meeting legislative and regulatory requirements.
To assess the safety performance of licensees, the CNSC conducts regulatory oversight activities including inspections, reviews of reports submitted by licensees, reviews of events and incidents, and general communication and exchanges of information with licensees.
CNSC staff use many metrics to evaluate licensees’ safety performance. This report uses a subset of these which – when taken together – have been determined to provide a well-rounded picture of performance for the licensees covered by this report. The metrics used in this report are:
- compliance performance
- enforcement actions
- doses to workers
- reported events
CNSC staff use a well-established Safety and Control Area Framework in evaluating each licensee’s safety performance. The framework includes 14 safety and control areas (SCAs) covering all technical areas of regulatory oversight. While CNSC staff review and assess performance in each SCA (if applicable), only those that are most useful in providing a good overall indication of the safety performance of the licensees are covered in the ROR, as explained below.
For Part I, the ROR will cover SCA ratings for management system, operating performance, radiation protection, and security. In addition, it will provide the Commission information about the effort on the part of CNSC to regulate these licensees, along with stakeholder engagement.
For Part II, the ROR will include ratings for all 14 SCAs and will focus on management system, radiation protection, and conventional health and safety SCAs for both TRIUMF and the Canadian Light Source for calendar years 2018 and 2019. Part II will also discuss CNSC's regulatory oversight and the facilities' public information and disclosure programs.
For both parts of the ROR, the main body of the report is meant to provide a high-level overview of the CNSC’s regulatory efforts, along with the licensees’ performance. The detailed data to support this overview can be found in the appendices: appendices A to F cover data linked to Part I of this report; appendices G to L link to Part II; and appendices M to P provide generic information applicable to both.
Part I: Use of Nuclear Substances in Canada: 2019
The Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2019 summarizes the safety performance of 1,494 licensees, which hold a total of 2,090 licences. Additional data on licensees is available in Appendix A. The CNSC authorizes these licensees to use nuclear substances and prescribed equipment in the medical, industrial, academic and research, and commercial sectors. Waste nuclear substance licences are included within the commercial sector. Section 4 of the 2018 ROR describes the sectors covered in this report.
For the purpose of Part I, CNSC staff evaluate safety performance by presenting licensees’ regulatory compliance in select Safety and Control Areas (SCAs) (that is, management system, operating performance, radiation protection and security), as well as enforcement actions, effective doses to workers and reported events.
In 2019, as part of the ongoing regulatory oversight of licensees, CNSC staff conducted compliance verification activities consisting of field inspections, desktop reviews and technical assessments of licensee activities. The evaluations of findings for the SCAs covered in Part I show that, overall, licensees made acceptable provision to protect health, safety, security, and the environment from the use of nuclear substances and prescribed equipment, and took the measures required to implement Canada’s international obligations. Based on these evaluations, CNSC staff conclude that the use of nuclear substances and prescribed equipment in Canada remains safe and secure.
I.1 Compliance Performance
During licensing and compliance activities, CNSC staff review the licensee’s (or applicant’s) performance within each relevant SCA by reviewing licensee documents and conducting inspections. Owing to the broad nature of the different activities conducted by the licensees covered in Part I, not all SCAs apply to all activities or all licensees. Although not incorporated into Part I, all relevant SCAs are assessed during compliance inspections and reviews of licensees’ documents, and a compliance rating is assigned for each SCA. Each SCA covers multiple items: some of these are administrative in nature and are considered relatively low risk, while others are linked to an immediate risk to health safety and security, and therefore any findings against these items during an inspection must be addressed immediately. Licensees are given a below expectation rating in an SCA if they are non-compliant in at least one item of an SCA, regardless of the risk.
All required corrective actions arising from below-satisfactory performance are tracked and followed up by CNSC staff to ensure that all items of non-compliance are addressed to the satisfaction of the CNSC. For any instances of non-compliance that pose immediate risk to health, safety and security enhanced enforcement actions, such as orders or Administrative Monetary Penalties, may be taken.
In Part I, the performance of a subset of the SCAs evaluated during inspections is reviewed and reported. The following four SCAs are the most relevant indicators of safety performance for licensees in the sectors covered in Part I: management system, operating performance, radiation protection and security. These SCAs are applicable to most of the licensees covered by Part I and, together, provide an indication of licensees’ overall safety performance. Appendix M provides a description of the rating framework.
In 2019, CNSC staff conducted 863 inspections across the four sectors. Overall, licensees showed satisfactory compliance ratings in all of the SCAs examined in Part I. A list of inspections performed in 2019 is available in Appendix F. Where items of non-compliance were identified, CNSC staff ensured that licensees took appropriate corrective actions. Licensees immediately addressed any items of non-compliance that had immediate risks to health, safety or security.
I.1.1 Management System
In 2019, licensees continued to maintain strong performance in the management system SCA. Overall, 97% of the licensees inspected received ratings of fully satisfactory or satisfactory for this SCA). The management system SCA has been performing with similar results over the past few years.
Refer to Appendix B.1 for additional information.
I.1.2 Operating Performance
In 2019, licensees’ performance in the operating performance SCA improved from previous years. This is a reversal from the previous years that showed a slow decline. Of the licensees inspected, 86% received ratings of fully satisfactory or satisfactory for this SCA.
The only decline was in the commercial sector, although the overall results for this sector are still at 89% fully compliant. For context, this means four of the 36 licensees inspected in the commercial sector had a non-compliance in this SCA, none of which were a concern. In creating its inspection plan for 2019, DNSR prioritized its most overdue medium risk licensees over well performing high risk licensees. Because of this approach, a reduction in performance in the medium risk use types, including those in the commercial sector, was expected. This is the first year a decline in this sector was observed; this will be evaluated in upcoming years to see if this decline is a trend or single occurrence.
Refer to Appendix B.2 for additional information.
I.1.3 Radiation Protection
Licensee’s overall performance in the radiation protection SCA declined in 2019, with 80% of licensees receiving ratings of fully satisfactory or satisfactory. The decreases were all medium risk licensees.
As with the operating performance SCA, the increase in the number of licensees with below expectations ratings in the radiation protection SCA may be due to prioritizing medium risk licensees that were overdue for inspection, particularly within the medical sector and the industrial sector, especially portable gauge licensees. The focus was on licensees that had not been inspected in the last five years, licensees with a poor compliance history and new licensees that had not been inspected. This focus provides a possible rationale for the observed decline in performance across this SCA. In addition, there was an increased focus from the CNSC inspectors to look at the licensee’s implementation of the radiation protection program and management oversight of their operational activities, especially for licensees that have not been inspected for some time. This may also have contributed to the increased number of non-compliances in that area and, therefore, contributed to the overall decline in performance across this SCA. Both the medical licensees and the portable gauge licensees were highlighted in the case studies provided in the 2018 ROR. The regulatory strategy described in those case studies was to target these licensees using a performance-based inspection, observing workers performing their duties. This inspection approach has improved detection of common areas of non-compliance, which is another possible explanation for the perceived decline in performance in this SCA.
The performance of the radiation protection SCA will continue to be monitored in order to determine if additional actions are required. The inspection frequency for medium risk licensees is every two to five years so it may take a few years before improvement is noticed on subsequent inspections.
Refer to Appendix B.3 for additional information.
I.1.4 Security
In the security SCA, 95% of the licensees inspected demonstrated that they have adequate provisions in place to prevent the loss, sabotage, illegal use, illegal possession or illegal removal of nuclear substances and prescribed equipment in their care and control. This SCA performance is up almost 5% in 2019 from the 2018 and 2017 results. The 2019 results are trending at similar levels to 2015 and 2016. This is in keeping with the analysis in the 2018 ROR, which predicted a temporary period of lower performance as licensees adjusted to the new security requirements in REGDOC-2.13.2.
Refer to Appendix B.4 for additional information.
I.2 Enforcement
The CNSC uses a graduated approach to enforcement to encourage compliance. When non-compliance (or continued non-compliance) has been identified, CNSC staff assess the significance of the non-compliance and determine the appropriate enforcement action.
In 2019, the CNSC initiated 13 escalated enforcement actions against licensees in the four sectors. There were no administrative monetary penalties (AMPs) issued in 2019. Most of the enforcement actions were taken against licensees in the industrial sector, consistent with trends from previous years. Out of 13 enforcement actions, 10 are closed as of the drafting of this ROR, meaning the CNSC is satisfied that the licensee has addressed the conditions of the order. Any high risk or immediate health and safety findings were immediately addressed. In three cases, licensees that received orders have yet to comply with the terms and conditions of their orders and the orders remain open. The CNSC is actively working with the licensees in these orders to ensure that order requirements are addressed. Refer to Appendix C for further details on enforcement actions taken in 2019.
I.3 Effective Doses to Workers
Licensees are required to keep radiation doses to persons below regulatory limits and ALARA in accordance with the radiation protection programs established under the CNSC licences.
In 2019, doses were monitored for 63,015 workers in the four sectors covered in Part I. Of those workers, 26,539 were classified as nuclear energy workers (NEWs). The remaining 36,476 were not classified as NEWs and are referred to as non-NEWs in the report. Exposures to radiation continued to be very low for workers in 2019, consistent with previous reporting years. Additional information on effective doses to workers is in Appendix D.
In 2019, one NEW received a whole body dose above the regulatory limit of 50 mSv. The CNSC was notified that a worker received a dose of 57 mSv in November 2019. CNSC staff presented information on this event at the Commission hearing in June 2020. More information on this event can be found in Appendix E.
One non-NEW received a whole body dose above the regulatory limit of 1 mSv. More information on this event can be found in Appendix E.
In both of these cases, the doses received do not pose a risk of radiation-related health effects.
I.4 Reported Events
CNSC staff assessed the 183 eventsFootnote 1 reported by licensees covered in Part I. Reported events have been ranked using the International Nuclear and Radiological Event Scale (INES). Of these, 181 were ranked as level 0 (no safety significance), one was ranked as level 1 (anomaly) and one was ranked as level 2 (incident). These are the same events described in the previous section on Effective Doses to workers. The INES level 1 event is the non-NEW that received a whole body dose above the regulatory limit of 1 mSv. The INES level 2 event is the event where the NEW received a whole body dose above the regulatory limit of 50 mSv. Refer to Appendix E for the list of reported events.
For all of the events reported, licensees implemented appropriate response measures to mitigate the impacts of the events and to limit radiation exposure to workers and the public. CNSC staff reviewed the measures and found them to be satisfactory.
I.5 Case Studies in Regulatory Intervention
CNSC staff monitor the performance of licensees across all sectors, using various metrics. When
these metrics indicate that the performance is not meeting expectations, or is declining, CNSC staff develop
regulatory strategies to address the situation. Once the strategies have been implemented, CNSC staff monitor their
effectiveness and adjust them if necessary.
Two case studies on licensees from the commercial sector are presented in this ROR. The first is a case study on an Iodine-131 (I-131) Processing licensee, Isologic Innovative Radiopharmaceuticals Ltd. (IIR), and the second is on a Positron Emission Tomography (PET) isotope cyclotron and associated processing laboratories, Montreal Neurological Institute and Hospital. The case studies are not intended to be punitive, they are intended to demonstrate lessons learned from a regulatory oversight perspective and actions taken by licensees that resulted in improvements in their programs. These lessons could be implemented by other licensees.
I.5.1 I-131 Processing Facility
I.5.1.1 Introduction
I-131 is a radioisotope that has been used in the Nuclear Medicine industry for decades. It is used to diagnose and treat thyroid disorders, as well as to diagnose certain neuroendocrine tumours in both adults and children. I-131 is also used in veterinary medicine, particularly for the treatment of feline hyperthyroidism.
Production of I-131 radiopharmaceuticals is considered a high risk activity due to the volatile nature of iodine. Airborne I-131 is easily concentrated in the thyroid, so appropriate containment measures must be used. Due to this high risk nature, there are only a small number of radiopharmaceutical processing facilities in Canada. This case study looks at the decline in safety performance at one such facility, Isologic Innovative Radiopharmaceuticals Ltd. (IIR), and CNSC staff’s subsequent increase in regulatory oversight, thereby ensuring the protection of the public and the environment.
I.5.1.2 Background
This licensee has been expanding its operations in Canada for some years. In a short length of time, they increased the number of licensed locations under their processing licence from one location to seven and introduced the manufacturing of I-131 capsules to the services that they provide to their customers.
Over the course of a four-year period, from 2014 to 2018, CNSC staff observed a significant decline in safety performance, which began with the licensee reporting to the CNSC a series of events in 2014 involving contaminated packages that were delivered to multiple hospitals. In reviewing these events, CNSC staff concluded that, although these incidents were of minor safety significance due to the relatively low levels of contamination involved, the licensee did not have sufficient management oversight of their work practices. The RSO at the time committed to improve the management oversight practices within their organization.
In February 2017, CNSC staff conducted a Type I inspection of the licensee’s operations, which span locations across Canada; the results of this inspection demonstrated a further decline in performance. The concerns were related to the licensee’s management oversight of the radiation protection program, more specifically towards the I-131 manufacturing operations. The CNSC performed two follow-up inspections in May and October 2017. The licensee worked through the implementation of the corrective actions following these inspections.
In the fall of 2018, the licensee reported to the CNSC two events at one of their locations. The first occurred in November, when a nuclear energy worker (NEW) exceeded the equivalent dose limit to the skin of the hand while conducting therapeutic I-131 capsule production. A few days after the event, CNSC staff conducted an inspection at the licensee’s location. The inspection found several non-compliances with regulatory requirements, which included workers routinely not following procedures relevant to I-131 processing and personnel monitoring. In addition, CNSC staff concluded that the responsibilities for implementation and oversight of the Radiation Protection Program were not clearly understood and executed by licensee staff. The licensee reported the second event one month later, indicating that they had exceeded their quarterly and annual airborne release limits for I-131. CNSC staff presented the reported events and inspection findings to the Commission in CMD 18-M65 as an Event Initial Report on December 13, 2018. Six days later, a CNSC Designated Officer (DO) issued an order to the licensee to immediately cease all processing of I-131 until such time that actions are taken to train staff, conduct a root cause analysis on I-131 releases and establish a program to monitor workers’ adherence to procedures. In addition, the order required the licensee to immediately make provisions to ensure adequate supply of I-131 radiopharmaceutical products to Canadian patients throughout the time period of suspended processing. CNSC staff reported this DO order to the Commission and continued to provide updates to the Commission on several occasion in the 14 months that followed the issuance of the Order.
CNSC staff conducted two additional compliance inspections in 2019; based on the results of these inspections, CNSC staff determined that the licensee had met all the terms and conditions of the Order. As previously reported to the Commission, the Order was closed on January 7, 2020.
I.5.1.3 Regulatory Approach
The standard CNSC approach to regulatory oversight for licensees using nuclear substances and prescribed equipment focuses on regulating similar activities across multiple licensees. However, in the case of Isologic, which is the biggest provider of I-131 to the medical sector in Canada, CNSC staff recognized that due to the size of its operations, this licensee required a tailored regulatory oversight strategy.
In addressing the situation described through this case study, the CNSC took a much more active role in the review of the licensee’s procedures than is typically required with this type of licensee. The goal of CNSC staff was to assist the licensee to significantly improve their programs to prevent reoccurrence of similar events moving forward. The regulatory approach included regular progress meetings with the licensee, as well as a detailed review and oversight of the environmental protection program. CNSC staff used the expert knowledge of subject matter specialists including staff from radiation protection, environmental protection, licensing, and compliance, to review the licensee’s submissions and to provide recommendations to the licensee. The licensee implemented a number of corrective actions, which the CNSC closely monitored through a phased approval process with regulatory hold points.
In the end, this team-based approach determined that the licensee’s submissions addressed the causes of the non-compliances that resulted in the issuance of the DO Order, and that the restart of I-131 operations would continue to protect the environment, the health and safety of workers, and the public.
I.5.1.4 Conclusion
At this time, the licensee remains fully compliant in their I-131 operations and continues to work closely with the CNSC to implement continuing improvements to their management system within their organization. The CNSC is planning a further compliance inspection of this licensee before the end of 2020 to verify the implementation of additional commitments that have been made.
I.5.2 Positron Emission Tomography (PET) Isotope Cyclotron Facility
I.5.2.1 Introduction
Isotope production accelerators such as cyclotrons produce isotopes used in the synthesis of nuclear medicine radiopharamaceuticals, used primarily for nuclear medicine imaging. Isotope production licensees may produce isotopes for both clinical and research purposes.
The licensee examined in this case study, the Montreal Neurological Institute and Hospital, has been running a Positron Emission Tomography (PET) isotope cyclotron and associated processing laboratories for almost three decades. There are two laboratories in the facility: one to handle clinical production of Fluorodeoxyglucose (FDG), a radiopharmaceutical used in medical imaging, in a fully automated manner inside shielded cells (hotcells); the second is for making various isotopes and tracers used for research in a nearby hospital.
At this facility, processing isotopes in the research lab is not fully automated. While most of the processing is done automatically, certain steps involve handling of vials or syringes inside the hotcells. This is because the hotcells are not equipped with robotic arms or remote handling manipulators. This means workers use their hands in the process to handle vials or other samples through small arm doors in the hotcell front shielding.
I.5.2.2 Background
Action levels are intended to provide an early indication of a possible loss of control of part of a licensee’s radiation protection program. In January and February of 2017, the licensee reported to CNSC staff that the monthly extremity dose action levels of 450 mSv/y (extrapolated monthly) were exceeded for personnel processing radioisotopes. As this dose level was still below the regulatory equivalent dose limit of 500mSv/y, the licensee was still compliant with the Radiation Protection Regulations.
As part of routine regulatory oversight, CNSC staff performed a benchmark analysis of worker doses as a function of the annual production for 17 isotope production cyclotron licensees in Canada. The purpose of this analysis was to evaluate licensee performance in this sector by normalizing dose based on total production at the facility and the number of workers, taking into account the nature of the operation: research vs commercial production. The benchmarking analysis showed that this particular licensee’s workers received the highest extremity annual doses normalized to the facility annual production compared to similar licensees.
Prompted by these two indicators, CNSC staff decided to take additional actions, starting with enhanced monitoring of the licensee. An October 2017 inspection revealed that the licensee had made little progress toward reducing the collective extremity dose. CNSC staff requested that the licensee review all radiochemistry processes and begin monthly reporting outlining their progress toward the goal of reducing extremity doses.
The licensee complied with the CNSC staff request and subsequently committed to two improvements to help reduce extremity doses: namely, using special tools to increase the distance between the source and the fingers, and semi-automating certain setups to reduce handling. A follow up inspection in March 2019 indicated that the licensee was not following its own internal procedures.
In order to mitigate the risk of continued high extremity doses, a CNSC inspector issued an order to the licensee to prohibit any handling inside the hotcell of activity in excess of 11.1 GBq (300 mCi) until the licensee submitted adequate processes for minimizing worker doses. CNSC staff ultimately determined that the licensee had met all the terms and conditions of the Order, and the Order was subsequently closed on November 5, 2019.
I.5.2.3 Regulatory Approach
In addition to assessing licensee performance through standard compliance activities, such as conducting inspections and reviewing annual compliance reports, CNSC staff also monitor other indicators that help to flag possible areas of concern. In this case, a review of events reported by this licensee, combined with a benchmarking analysis of worker doses, led CNSC staff to increase its regulatory oversight of this licensee through the application of a number of compliance tools, applied in an escalated manner. As a result of these regulatory actions, the licensee improved its performance with respect to radiation safety.
I.5.2.4 Conclusion
The licensee has demonstrated a clear reduction of extremity doses: the maximum yearly extremity doses decreased from 348 mSv in 2018 to 196 mSv in 2019. CNSC staff continue to monitor licensee performance through a combination of desktop compliance verification and on-site inspections to ensure sustained good performance.
I.6 Stakeholder Engagement
Nuclear substance licensees are not required to have public information disclosure programs (unlike the Class IB Accelerator licensees covered in Part II of this report). Stakeholder engagement and outreach activities are performed by the CNSC to facilitate communication on licenced activities and regulatory expectations between the CNSC, nuclear substance licensees, the public, and Indigenous communities.
Stakeholder engagement and outreach are critical elements of the CNSC’s regulatory approach. Given the breadth of licensees regulated in the area of nuclear substances, a particular focus is on reaching and engaging with licensee communities, which leads to increased awareness and better understanding of the regulatory process and requirements. CNSC staff leverage a variety of fora to engage with licensees and promote the use of the tools that are developed to support their compliance with regulatory expectations. Inspections are a particularly valuable opportunity to engage directly with licensees.
CNSC outreach in 2019 included:
- Annual meetings with the radiography industry: The CNSC holds two separate annual meetings with the radiography industry. In 2019 the meetings were held in Ottawa, Ontario on May 8th and Nisku, Alberta on May 23rd. CNSC staff use these meetings to address recent and upcoming regulatory developments and discuss other areas of regulatory focus.
- Newsletter: One issue of the DNSR Newsletter was published in the summer of 2019.
-
Working Groups:
- There were two meetings with the Industrial Radiography working group one in February and one in October 2019. The working group meets to discuss best practices and safety performance, and provides a forum in which stakeholders can stay informed of new developments from both technical and regulatory perspectives.
- There was also a meeting with the CRPA working group during the CRPA conference in May 2019. Topics for such meetings are varied, focusing on items of mutual interest, and are intended to foster open communications between CRPA and the CNSC. Topics have included: multi modality dose consideration in room approvals, designation of workers as NEWS without dose justification, discussion of upcoming regulatory documents and changes to the disposal table on licence.
- Industry meetings: CNSC staff met with the Canadian Council of Independent Laboratories in May 2019. CNSC Staff presented on trends in the portable gauge industry.
- Meetings with Indigenous groups: CNSC staff participated in Indigenous engagement outreach in Ontario and New Brunswick.
- Participation in CRPA meeting: CNSC staff delivered presentations at the Canadian Radiation Protection Association’s annual meeting in May 2019.
- Presentation to school students: CNSC staff participated in a STEM talk with students at a local Ottawa school, organized by Accelerator and Class II Facilities Division staff.
- Participation in COMP meeting: CNSC staff presented at the Canadian Organization of Medical Physicists annual scientific meeting in September 2019.
- Town Hall: CNSC staff held a virtual town hall meeting for Accelerator and Class II Facilities licensees. The Class II regulations were under review and changes to these regulations were discussed. There were 58 participants in this meeting.
I.7 Conclusion
In 2019, the majority of the inspected licensees were in compliance with the expectations of the SCAs in this report. Most of the enforcement actions taken in 2019 have been closed with the CNSC closely monitoring the three orders that are still open. Exposure to workers in 2019 continue to be very low and consistent with previous years. For the events reported in 2019, the licensees implemented appropriate responses to address the events, as determined by CNSC staff. CNSC staff continue to host outreach with various stakeholders to keep the public, Indigenous communities and licensees informed.
Through the case studies highlighted in this report the CNSC was able to improve compliance by helping licensees to focus on the right corrective actions to improve their performance.
Based on the CNSC’s comprehensive regulatory oversight of the industry, CNSC staff conclude that the use of nuclear substances and prescribed equipment in Canada is safe. Licensees corrected identified items of non-compliance to the satisfaction of CNSC staff; adequate provisions are in place to protect the health, safety and security of persons and the environment from the use of nuclear substances and prescribed equipment.
Part II: Class IB Accelerators in Canada: 2018-2019
The DNSR ROR: Part II presents the operating performance of the two Class IB accelerator facilities regulated by CNSC: the Tri University Meson Facility (TRIUMF) and the Canadian Light Source Inc. (CLSI).
TRIUMF operates one 520 megaelectronvolt (MeV) cyclotron accelerator facility, four smaller cyclotrons facilities, and three linear accelerator facilities. TRIUMF is located on the University of British Columbia campus in Vancouver, British Columbia. TRIUMF is a nuclear and particle physics research centre and is a major producer of radioisotopes used for medical diagnostic procedures. The 520-Mev cyclotron accelerator has been in operation since 1975.
CLSI operates a synchrotron facility, on the University of Saskatchewan campus in Saskatoon, Saskatchewan. The facility produces synchrotron radiation that is used as a light source for experiments in diverse fields. The facility has been in operation since 2005.
Public concern tends to be low regarding the safety performance of Class IB accelerator facilities. Since CNSC staff have been reporting the safety performance for the facilities it regulated (starting in 2013) the consultation process for the Class 1B accelerator ROR has never yielded comments from the public nor requests for participation at the Commission meetings. Therefore, the ROR on Class IB accelerators in Canada has been presented every second year since 2016.
II.1 Compliance Performance
The main hazards associated with Class IB accelerator facilities are radiological exposure and conventional industrial hazards. Nuclear substances are present as a result of deliberate irradiation of targets designed to produce desired isotopes, or as an unavoidable by-product generated in irradiated air or accelerator components. Consequently, TRIUMF`s environmental releases are very small and CLSI has no radioactive environmental releases resulting from the operation of the synchrotron.
CNSC staff conducted consistent and risk-informed regulatory oversight at the Class IB particle accelerator facilities. Table 20 of Appendix G presents the licensing and compliance efforts from CNSC staff for the Class IB accelerator facilities for 2018 and 2019.
During this two-year period, CNSC staff conducted six targeted onsite inspections, three at TRIUMF and three at CLSI. CNSC staff provided the findings from these inspections to the licensees in detailed inspection reports. Licensee corrective actions enacted in response to Notices of Non Compliance identified by CNSC staff are followed through to resolution.
These facilities are required, as part of their operating licences, to submit an annual compliance report (ACR) by June 30 each year. The review of these ACRs by CNSC subject matter experts complements the compliance inspections and provides verification of licensee performance on an annual basis.
In comparison with previous years, regulatory efforts increased slightly in 2018 and 2019. This was primarily due to increased assessment efforts in response to necessary changes for both facilities in order to implement CSA Standard N286-12, Management System Requirements for Nuclear Facilities, which came out in 2012. Compliance with this standard was required starting January 2018 for TRIUMF and CLSI. This is discussed further in the Management System section below.
CNSC staff use the SCA framework in evaluating each licensee's safety performance level. Each SCA is rated through compliance inspections, desktop reviews of events and incidents, and ACR review. These ratings are used as an indicator of performance and potential areas requiring attention from the licensee and CNSC staff. Compliance oversight plans are developed by CNSC staff, taking into consideration a number of factors, including these ratings. A facility performing below the satisfactory level in any of the SCAs will be subject to escalated enforcement by CNSC staff until the situation is remedied.
For calendar years 2018 and 2019, the performance in all 14 SCAs for TRIUMF was rated as satisfactory or better, with the exception of the Management System SCA (as discussed in section II.1.1.1). For the same time period, CLSI’s performance was rated satisfactory or better in all SCAs, with the exception of the Management System SCA in 2019 (as discussed in section II.1.1.2). Appendix H provides the SCA ratings for the past five years for TRIUMF (Table 14) and CLSI (Table 15).
II.1.1 Management System
The Management System SCA covers the framework which establishes the processes and programs required to ensure an organization achieves its safety objectives and continuously monitors its performance against these objectives and fostering a healthy safety culture.
Since January 2018, Class IB accelerator facilities are required to comply to CSA Standard N286-12, Management System Requirements for Nuclear Facilities, as stated in the licence condition handbook.
II.1.1.1 TRIUMF
In February 2016, CNSC staff carried out a management system inspection at TRIUMF. TRIUMF took corrective actions to address the findings from that inspection to CNSC satisfaction. Following the inspection, TRIUMF committed to comply with CSA Standard N286-12 by January 2018. However, it took longer than expected for TRIUMF to perform a gap analysis of their management system against N286-12 and therefore TRIUMF did not meet the deadline. TRIUMF decided to increase resources committed to this task and spent most of 2019 looking for a suitable quality assurance (QA) manager responsible for coordinating these compliance efforts. CNSC staff delayed the February 2019 management system inspection due to TRIUMF's lack of progress on performing the gap analysis and closing the gaps. In December 2019, TRIUMF hired a new QA manager. In March 2020, TRIUMF's new QA manager submitted a gap analysis report to CNSC staff. CNSC staff reviewed the gap analysis and focussed communications between the new QA manager and CNSC management specialists have now put TRIUMF on the right track to demonstrate that they will meet N286-12 requirements by the end of 2020. CNSC staff rescheduled the February 2019 management system inspection for the fall of 2020.
TRIUMF operates under an effective management system, which allows them to perform their licenced activities safely. However, CNSC staff rated TRIUMF below expectations for the Management System SCA for both 2018 and 2019 because TRIUMF failed to demonstrate compliance to CSA Standard N286-12. TRIUMF’s gap analysis of their management system against the standard may result in opportunities for improvements that will increase the defence in depth, resulting in increased safety. The delay does not pose an immediate risk to health and safety.
II.1.1.2 CLSI
CLSI performed a gap analysis of their management system against the N286-12 standard and updated their procedures accordingly in order to meet the January 2018 compliance date. Upon initial review of their revised management system program, CNSC staff determined that CLSI was compliant. Subsequently, CLSI underwent a management system inspection in July 2019, focused on N286-12 implementation. CNSC staff noted that CLSI had made some progress in their management system. However, it was also noted that CLSI was not complying with its own governance and the problem identification and resolution process was found to be non-compliant with N286-12 requirements.
CNSC staff concluded that CLSI is partially meeting the N286-12 requirements. Despite these non-compliances, CLSI has an effective management system and continues to safely operate the Class IB facility. Further compliance activities are planned in 2020 in order to follow up with the deficiencies flagged and ensure that CLSI is in full compliance with N286-12. As a result, CLSI has been rated as below expectations for the Management System SCA for 2019.
II.1.2 Radiation Protection
The Radiation Protection SCA covers the implementation of a radiation protection program in accordance with the Radiation Protection Regulations. The program must ensure that contamination levels and radiation doses received by individuals are monitored, controlled and maintained ALARA.
The rating for the Radiation Protection SCA for both Class IB accelerator facilities was satisfactory or better, which is unchanged from the previous three years. The ratings for the past five years are presented in Appendix H.
During 2018 and 2019, CNSC staff determined that all Class IB accelerator facilities implemented effective measures to keep radiation exposures and doses to persons ALARA. This has consistently resulted in doses to persons being well below CNSC regulatory dose limits.
All Class IB accelerator facilities continued to comply with the regulatory requirements to measure and record doses received by workers. Details are provided in section II.3 below.
Both TRIUMF and CLSI have put in place dose action levels that, if reached, may indicate a loss of control of part of their radiation protection program. In 2018 and 2019, there were no occurrences of dose exceeding action levels at either facility.
Both TRIUMF and CLSI have put in place comprehensive radiation dose area monitoring programs and surface contamination monitoring and mitigation programs. CNSC staff routinely verify the results and compare them to previous years’ results. No anomalies were noted in 2018 or 2019.
Doses to members of the public from the Class IB facilities has been consistently well below the CNSC regulatory dose limit for a member of the public of 1 mSv/year. For TRIUMF, the public dose trend for the last five year is presented in Table 23 of Appendix I. There are no airborne or liquid effluent releases of nuclear substances from CLSI. Therefore, the estimated dose to the public is at natural radiation background levels for this facility.
CNSC staff conclude that for 2018 and 2019, the Class IB accelerator facilities effectively maintained their radiation protection programs to ensure the health and safety of persons present in their facilities.
II.1.3 Conventional Health and Safety
The Conventional Health and Safety SCA covers the implementation of a program to manage workplace safety hazards and to protect personnel and equipment.
CNSC's risk-informed analysis of both Class IB particle accelerator facilities concluded that conventional health and safety is the only SCA for which both the probability of an accident is high and the impact is high. Therefore, the frequency of inspection of this SCA is the highest for all SCAs, with an interval of one to two years for TRIUMF and two to three years for CLSI. CNSC staff verified conventional health and safety at TRIUMF during the April 2018 inspection and again during the April 2019 inspection. CLSI's last conventional health and safety inspection was in 2017.
A key performance indicator for the conventional health and safety SCA is the lost-time injury rate. The lost-time injury rate is presented in Table 24 of Appendix J for the last five years, along with a description of all lost-time injuries for 2018 and 2019 in Table 18 of the same appendix.
The rating for the conventional health and safety SCA was satisfactory or better for both Class IB accelerator facilities in 2018-2019.
CNSC staff conclude that for 2018 and 2019, the Class IB accelerator facilities effectively maintained their conventional health and safety programs to ensure the health and safety of persons present in their facilities.
II.2 Enforcement
The CNSC uses a graduated approach to enforcement to encourage compliance. When non-compliance (or continued non-compliance) has been identified, CNSC staff assess the significance of the non-compliance and determine the appropriate enforcement action.
In 2018 and 2019, neither TRIUMF nor CLSI had enforcement actions.
II.3 Effective Doses to Workers
All Class IB accelerator facilities continued to comply with the regulatory requirements to measure and record doses received by workers. During 2018 and 2019, all Class IB accelerator facilities monitored and controlled the radiation exposures and doses received by all persons present at their licensed facilities, including workers, contractors and visitors. Figure 18 and 19 of Appendix K provides the effective doses to nuclear energy workers for Class IB accelerator facilities for this reporting period.
II.4 Reported Events
For 2018-2019, TRIUMF had 11 eventsFootnote 2 and CLSI had six events. Appendix L describes these events in detail. For each event reported, the licensees performed an internal investigation and implemented corrective actions to prevent reoccurrences. Both facilities reported these events to CNSC as required by the regulations or the licence conditions. In each case, CNSC staff reviewed the report and corrective actions and found them to be satisfactory.
II.5 Public Information and Disclosure Program
Class IB accelerator facilities have a responsibility to inform the public about their nuclear facilities and activities. CNSC staff recognize that Class IB accelerators are low-risk facilities and that a full-scale public information program, as undertaken by larger nuclear facilities, is not warranted. However, the CNSC requires these licensees to provide open and transparent information to the public. The objective is to ensure that timely information about the health, safety and security of persons and the environment and other issues associated with the nuclear facility are effectively communicated.
The public information and disclosure program was established in December 2018 for TRIUMF and in September 2018 for CLSI.
CNSC staff verified through annual compliance reporting that public information and disclosure programs were being implemented satisfactorily during 2018-2019. The CNSC has provided feedback on their communications programs to CLSI and TRIUMF, including areas for improvement to ensure that the programs remain effective at communicating useful information about the health, safety and security of persons and the environment and other matters of public interest associated with these facilities.
II.6 Conclusion
Through regulatory oversight activities, CNSC staff confirmed that Class IB Particle Accelerator facilities in Canada continued to operate safely in 2018 and 2019. The regulatory oversight activities included inspections, review of reports submitted by licensees, event and incident review with follow-up, general communication and exchanges of information with the licensees.
For the three SCAs presented in detail in this report, CNSC staff concluded that both the radiation program and the conventional health and safety program were satisfactory. However, the implementation of CSA Standard N286-12 into the management system of both Class IB accelerator facilities has been a challenge during the reporting period. As a result, CNSC staff has increased their regulatory efforts to bring both facilities closer to full compliance with the standard.
CNSC staff conclude that for the reporting period, both TRIUMF and CLS made adequate provision for the health and safety of workers, the protection of the public and the environment, as well as Canada's international obligations.
Appendices A to F cover data linked to Part I of this report; appendices G to L link to Part II; and appendices M to P provide generic information applicable to both.
Appendix A: Regulatory Program for the Use of Nuclear Substances
This section presents additional regulatory data to complement the information provided in the main part of the document.
A.1 CNSC regulatory effort
| Type of decision | Number of decisions |
|---|---|
| Licensing (issuance of new licenses, licence renewals, licence amendments, licence revocations and licence transfers) | 1,780 |
| Certification of prescribed equipment (radiation devices, Class II prescribed equipment and transport packages) | 71 |
| Certification of exposure device operators (EDOs) (issuance of new certification and renewal of certification) | 498 |
| Certification of Class II RSOs | 9 |
| Total | 2,358 |
| Activity | Person-days |
|---|---|
| Licensing | 4,648 |
| Certification | 1,237 |
| Compliance verification | 6,061 |
A.2 Licensing
In 2019, there were 2,090 licenses held for the use of nuclear substances and prescribed equipment (Table 3). The licensees are located throughout Canada, as indicated in figure 1.
| Sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Medical | 494 | 470 | 457 | 436 | 438 |
| Industrial | 1,349 | 1,308 | 1,287 | 1,259 | 1,228 |
| Academic and research | 207 | 208 | 195 | 192 | 187 |
| Commercial | 251 | 254 | 252 | 248 | 237 |
| Total | 2,301 | 2,240 | 2,191 | 2,135 | 2,090 |
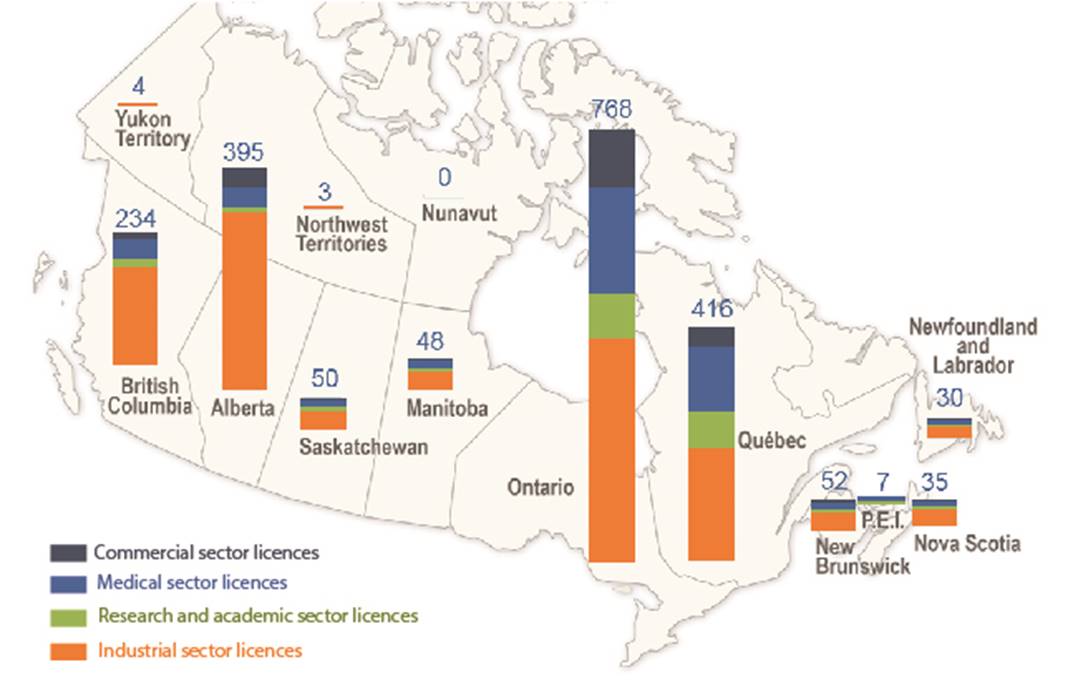
Figure 1: Text version
| Industrial | Academic | Medical | Commercial | |
|---|---|---|---|---|
| Alberta (AB) | 315 | 10 | 37 | 33 |
| British Columbia (BC) | 167 | 18 | 39 | 10 |
| Manitoba (MB) | 23 | 8 | 13 | 4 |
| New Brunswick (NB) | 30 | 5 | 14 | 3 |
| Newfoundland and Labrador (NL) | 21 | 1 | 6 | 2 |
| Northwest Territories (NT) | 3 | 0 | 0 | 0 |
| Nova Scotia (NS) | 23 | 3 | 5 | 4 |
| Nunavut (NU) | 0 | 0 | 0 | 0 |
| Ontario (ON) | 396 | 78 | 190 | 104 |
| Prince Edward Island (PE) | 2 | 1 | 4 | 0 |
| Quebec (QC) | 200 | 58 | 119 | 39 |
| Saskatchewan (SK) | 32 | 5 | 11 | 2 |
| Yukon (YT) | 4 | 0 | 0 | 0 |
A.3 Certification of prescribed equipment and transport packages
As seen in Table 1, designated officers made 71 decisions related to the certification of prescribed equipment (40) or transport packages (31).
A.4 Certification of exposure device operators
Licensees are required under the Nuclear Substances and Radiation Devices Regulations to permit only CNSC-certified personnel and supervised trainees to use exposure devices containing nuclear substances. In 2019, the CNSC certified 113 new exposure device operators (EDOs) and renewed the certifications of 385 others.
A.5 Certification of Class II radiation safety officers
All licensees that operate Class II nuclear facilities or that service Class II prescribed equipment must have a certified radiation safety officer (RSO) and a qualified temporary replacement. The RSO ensures that licensed activities are conducted safely and all regulatory expectations are met.
In 2019, the CNSC certified 9 Class II RSOs. No Class II RSOs were decertified in 2019.
Appendix B: Compliance Performance
B.1 Management System
For the management system SCA, 97% of the licensees inspected ensured that adequate processes and programs were in place to achieve their safety objectives (figures 2 and 3). Three licensees received unacceptable ratings in this SCA. There was one unacceptable results from the medical sector, one from the industrial sector and one from the commercial sector. For any unacceptable ratings, CNSC staff ensure that licensees took appropriate corrective actions. Licensees immediately addressed any items of non-compliance that had immediate risks to health, safety or security. The industrial licensee was issued an order(refer to order #1058 in Appendix C for further details). CNSC staff are monitoring the medical licensee’s progress for correcting the findings from the inspection. The commercial licensee has addressed all items of non-compliance and CNSC staff will further evaluate responses during the inspection scheduled in 2020.
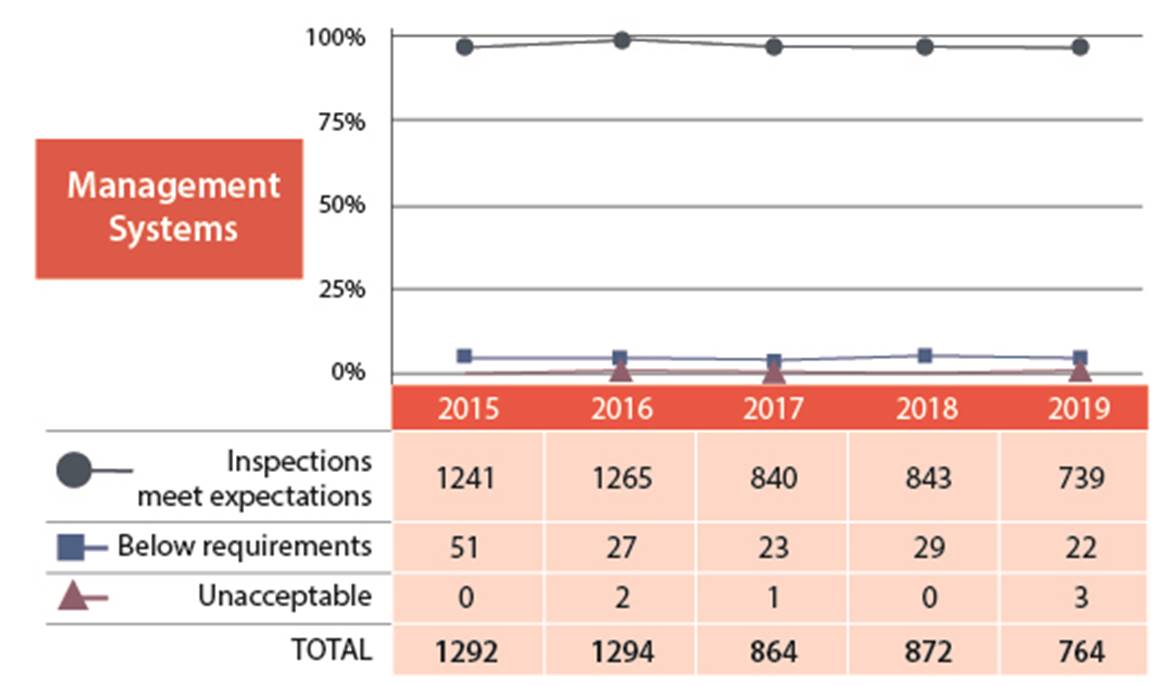
Figure 2: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Meets expectation | 1241 | 1265 | 840 | 843 | 739 |
| Below requirements | 51 | 27 | 23 | 29 | 22 |
| Unacceptable | 0 | 2 | 1 | 0 | 3 |
| Total | 1292 | 1294 | 864 | 872 | 764 |
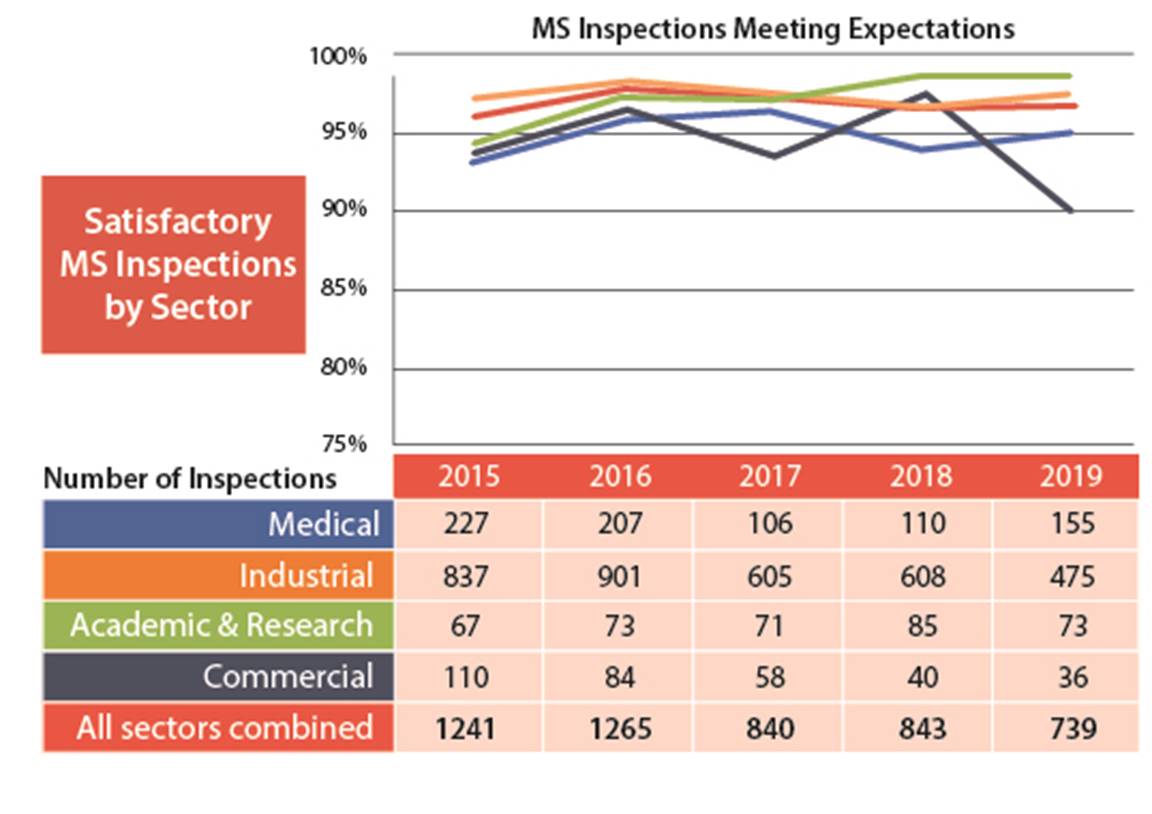
Figure 3: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Medical | 227 | 207 | 106 | 110 | 155 |
| Industrial | 837 | 901 | 605 | 608 | 475 |
| Academic and research | 67 | 73 | 71 | 85 | 73 |
| Commercial | 110 | 84 | 58 | 40 | 36 |
| All sectors combined | 1241 | 1265 | 840 | 843 | 739 |
B.2 Operating Performance
In the operating performance SCA, 86% of the licensees inspected made adequate provisions for health, safety, security, and the protection of the environment. In 2019, the operating performance SCA slightly improved from the 84% fully compliant, to 86%(figures 4 and 5).
Two licensees received an unacceptable rating in operating performance. One was from the industrial sector and one was from the academic and research sector. The industrial licensee was issued an order (refer to order #1112 in Appendix C for further details). The academic and research licensee has since implemented corrective measures that were reviewed by CNSC staff and were determined to be satisfactory.
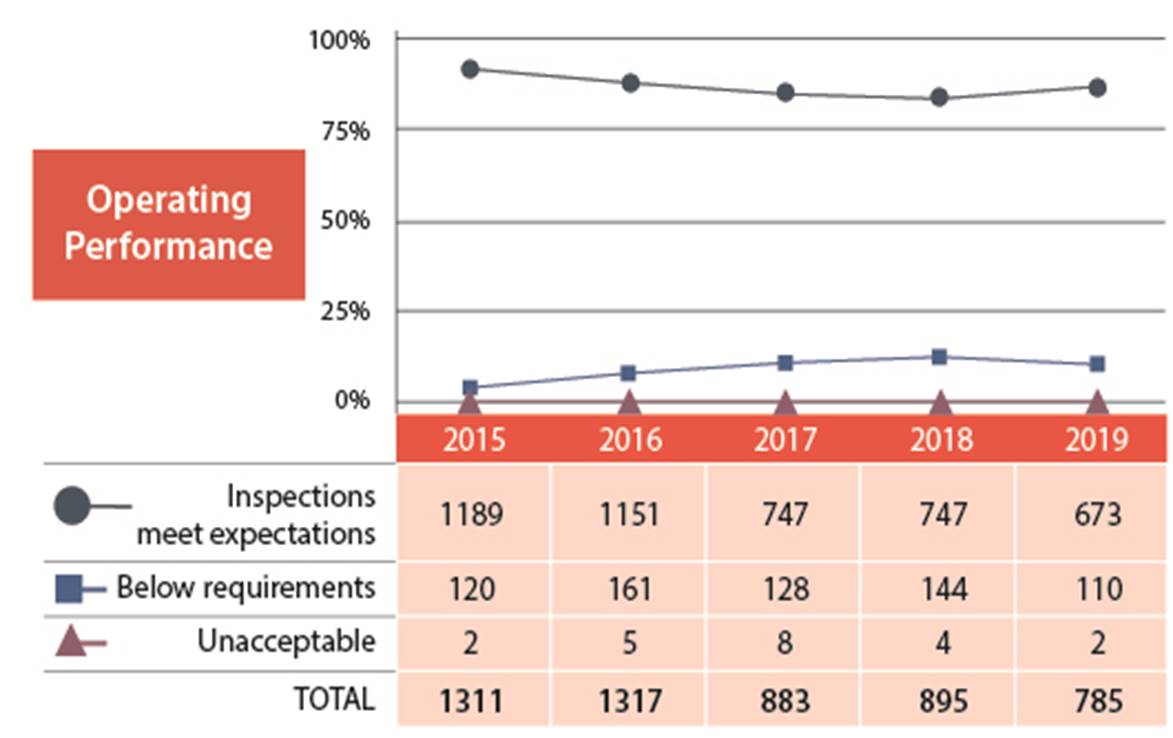
Figure 4: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Meets expectation | 1189 | 1151 | 747 | 747 | 673 |
| Below requirements | 120 | 161 | 128 | 144 | 110 |
| Unacceptable | 2 | 5 | 8 | 4 | 2 |
| Total | 1311 | 1317 | 883 | 895 | 785 |
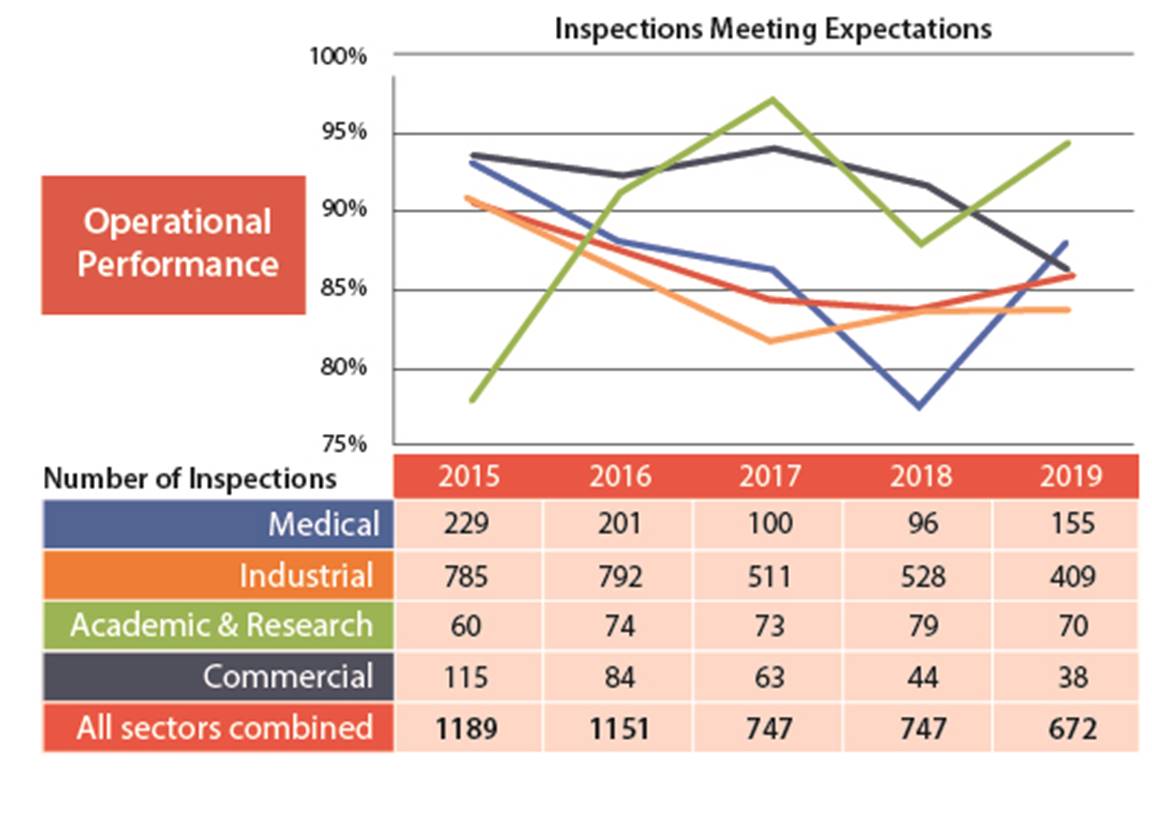
Figure 5: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Medical | 229 | 201 | 100 | 96 | 155 |
| Industrial | 785 | 792 | 511 | 528 | 409 |
| Academic and research | 60 | 74 | 73 | 79 | 70 |
| Commercial | 115 | 84 | 63 | 44 | 38 |
| All sectors combined | 1189 | 1151 | 747 | 747 | 672 |
B.3 Radiation Protection
In the radiation protection SCA, 80% of the licensees inspected had adequate measures and programs in place to ensure that exposure to workers and the public to ionizing radiation was monitored, and controlled, and remained ALARA (as low as reasonably achievable). Overall, this SCA has been trending downwards for the past few years from 89% meeting expectations in 2015 to 80% in 2019 (figures 6 and 7).
One industrial licensee received an unacceptable rating in radiation protection. The licensee was issued an order (refer to order #1207 in Appendix C for further details).
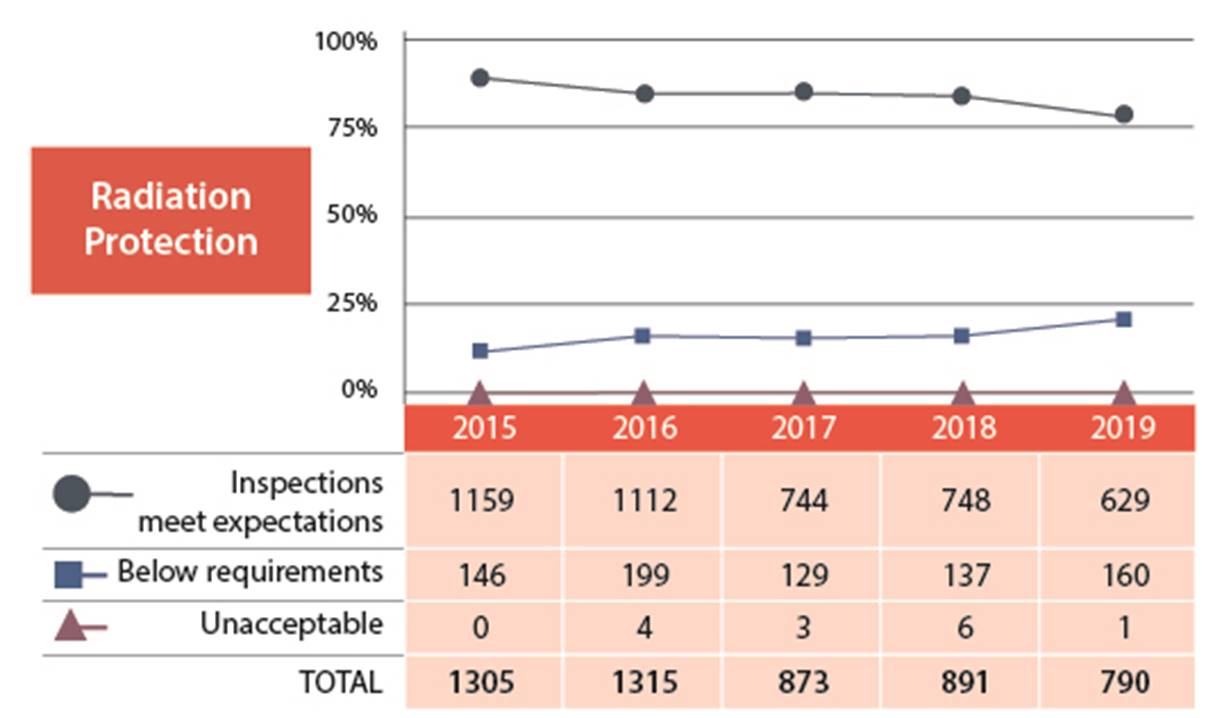
Figure 6: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Meets expectation | 1159 | 1112 | 744 | 748 | 629 |
| Below requirements | 146 | 199 | 129 | 137 | 160 |
| Unacceptable | 0 | 4 | 3 | 6 | 1 |
| Total | 1305 | 1315 | 873 | 891 | 790 |
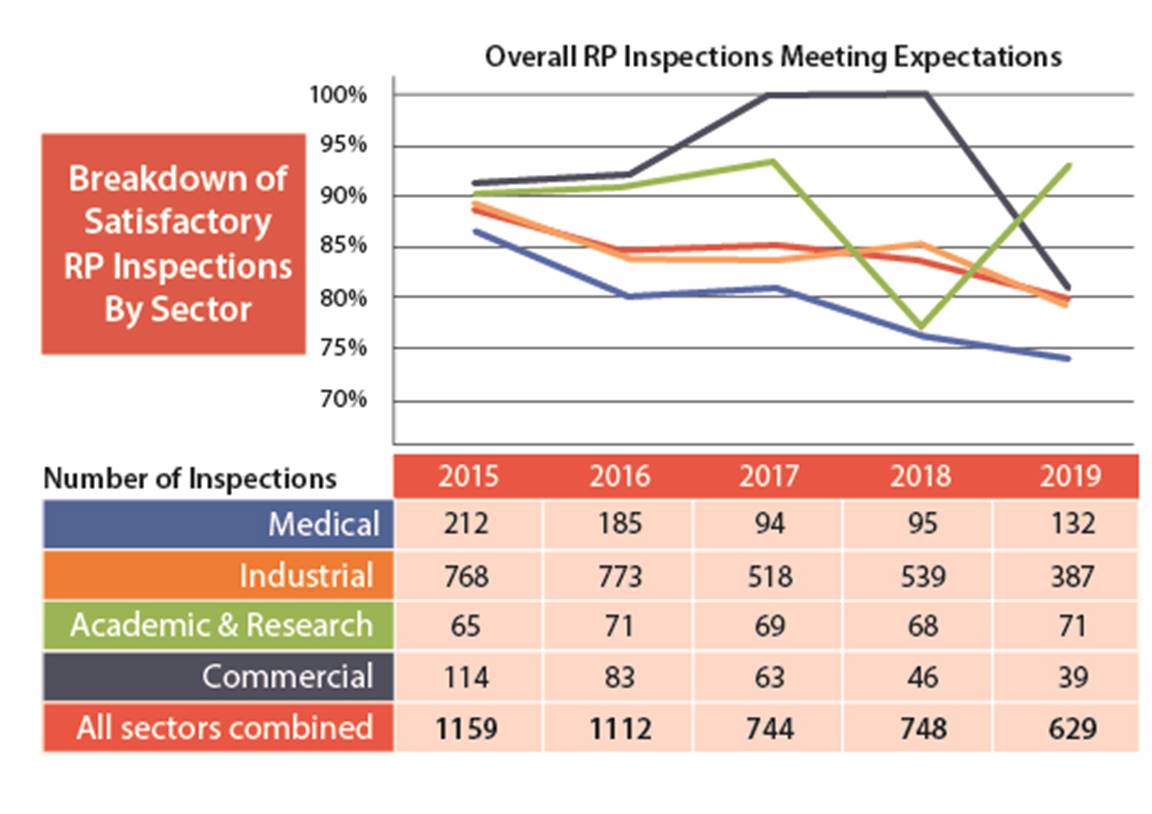
Figure 7: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Medical | 212 | 185 | 94 | 95 | 132 |
| Industrial | 768 | 773 | 518 | 539 | 387 |
| Academic and research | 65 | 71 | 69 | 68 | 71 |
| Commercial | 114 | 83 | 63 | 46 | 39 |
| All sectors combined | 1159 | 1112 | 744 | 748 | 629 |
B.4 Security
In 2019, licensees’ performance in the security SCA improved from previous years; 95% of inspected licensees (719 of 760) received fully satisfactory or satisfactory ratings (figures 8 and 9).
None of the licensees received an unacceptable rating for the Security SCA.
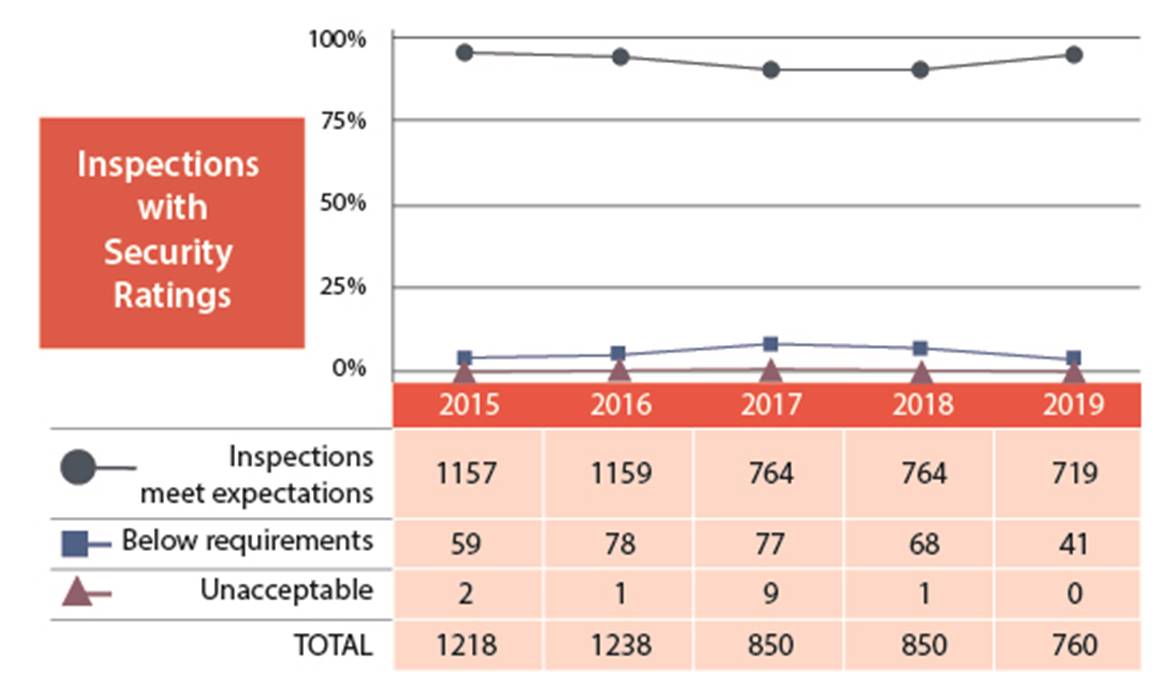
Figure 8: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Meets expectation | 1157 | 1159 | 764 | 764 | 719 |
| Below requirements | 59 | 78 | 77 | 68 | 41 |
| Unacceptable | 2 | 1 | 9 | 1 | 0 |
| Total | 1218 | 1238 | 850 | 850 | 760 |
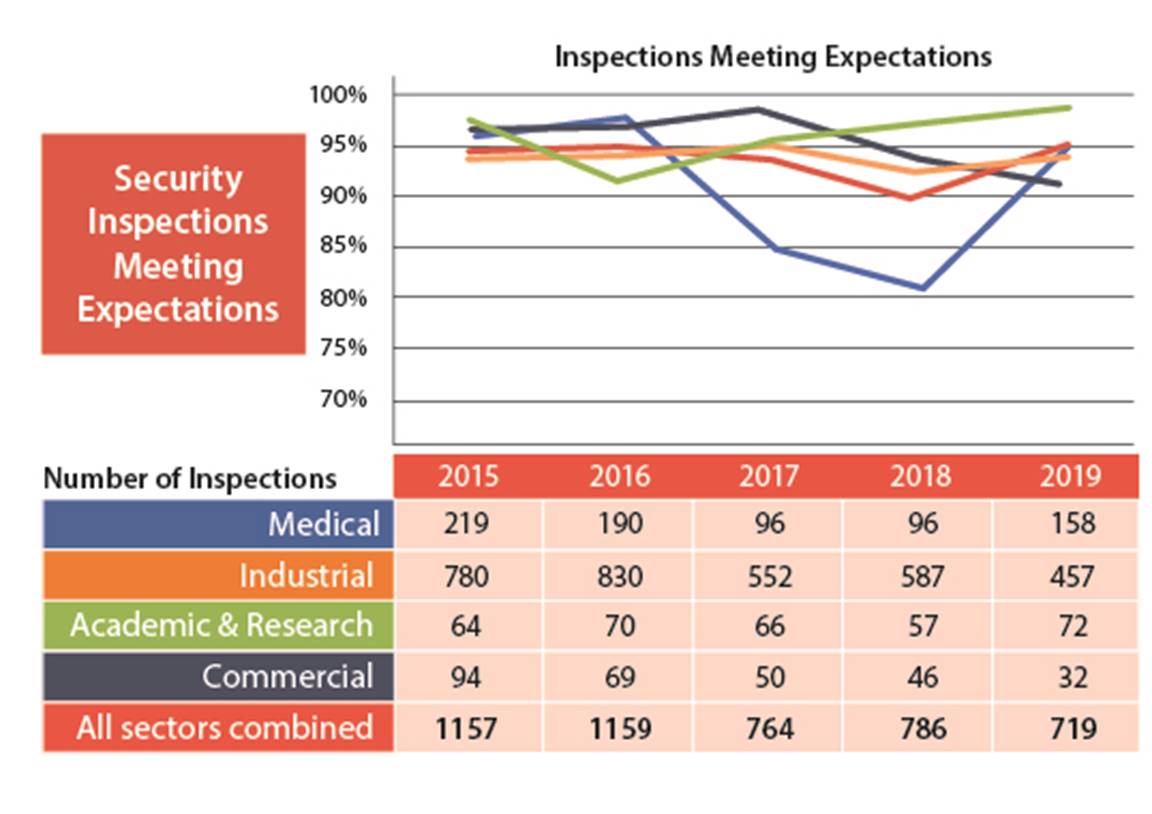
Figure 9: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Medical | 219 | 190 | 96 | 96 | 158 |
| Industrial | 780 | 830 | 552 | 587 | 457 |
| Academic and research | 64 | 70 | 66 | 57 | 72 |
| Commercial | 94 | 69 | 50 | 46 | 32 |
| All sectors combined | 1157 | 1159 | 764 | 786 | 719 |
B.5 Inspection rating, by sector
B.5.1 Medical sector
Tables 4 to 7 in this appendix shows the inspection performance of licensees in the medical sector. The performance of the subsectors is shown for the years 2015 – 2019 as a percentage of the inspections that received fully satisfactory or satisfactory grades for the SCA and the total number of inspections where performance in that SCA was assessed. The number of inspections for the medical sector is the aggregate for the entire sector, including subsectors not highlighted in Part I.
A breakdown by subsector is not provided for the security SCA given the potentially sensitive information associated with that SCA.
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Management system | Nuclear medicine |
93% (203) |
96% (174) |
98% (91) |
96% (103) |
95% (103) |
| Radiation therapy |
93% (14) |
67% (10) |
82% (11) |
50% (6) |
100% (4) |
|
| Veterinary nuclear medicine |
100% (6) |
100% (9) |
100% (4) |
100% (4) |
75% (4) |
|
| Medical sector |
94% (242) |
96% (216) |
97% (110) |
94% (117) |
95% (163) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Operating Performance | Nuclear medicine |
93% (205) |
86% (184) |
86% (90) |
77% (104) |
87% (155) |
| Radiation therapy |
93% (14) |
92% (24) |
89% (18) |
67% (12) |
100% (21) |
|
| Veterinary nuclear medicine |
100% (6) |
100% (9) |
100% (4) |
100% (4) |
100% (3) |
|
| Medical sector |
93% (246) |
88% (228) |
87% (116) |
77% (124) |
88% (176) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Radiation Protection | Nuclear medicine |
85% (205) |
77% (186) |
75% (89) |
74% (104) |
70% (155) |
| Radiation therapy |
100% (15) |
100% (24) |
100% (19) |
100% (12) |
100% (13) |
|
| Veterinary nuclear medicine |
83% (6) |
67% (9) |
100% (4) |
50% (4) |
100% (3) |
|
| Medical sector |
86% (246) |
80% (231) |
81% (116) |
77% (124) |
74% (178) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Security | Medical Sector |
98% (223) |
86% (222) |
81% (118) |
91% (119) |
94% (168) |
B.5.2 Industrial sector
Tables 8 to 11 in this appendix shows the inspection performance of licensees in the industrial sector. The performance of the subsectors is shown for the years 2015 – 2019 as a percentage of the inspections that received fully satisfactory or satisfactory grades for the SCA and the total number of inspections where performance in that SCA was assessed. The number of inspections for the industrial sector is the aggregate for the entire sector, including subsectors not highlighted in Part I.
A breakdown by subsector is not provided for the security SCA given the potentially sensitive information associated with that SCA.
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Management system | Portable gauge |
99% (338) |
98% (443) |
99% (303) |
98% (321) |
100% (215) |
| Fixed gauge |
96% (170) |
100% (205) |
94% (130) |
94% (112) |
94% (124) |
|
| Industrial radiography |
96% (163) |
97% (201) |
96% (136) |
96% (138) |
98% (114) |
|
| Oil well logging |
98% (50) |
100% (48) |
100% (42) |
98% (43) |
100% (24) |
|
| Industrial sector |
97% (860) |
98% (916) |
98% (620) |
97% (608) |
98% (487) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Operating Performance | Portable gauge |
92% (389) |
87% (439) |
82% (305) |
86% (326) |
82% (216) |
| Fixed gauge |
90% (170) |
77% (205) |
70% (136) |
68% (111) |
73% (124) |
|
| Industrial radiography |
92% (190) |
94% (199) |
89% (116) |
88% (138) |
93% (114) |
|
| Oil well logging |
77% (49) |
90% (48) |
93% (42) |
86% (44) |
100% (24) |
|
| Industrial sector |
91% (865) |
86% (917) |
82% (625) |
83% (633) |
83.9% (484) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Radiation Protection | Portable gauge |
91% (389) |
84% (442) |
82% (306) |
84% (326) |
74% (216) |
| Fixed gauge |
80% (170) |
78% (205) |
80% (132) |
77% (111) |
73% (124) |
|
| Industrial radiography |
92% (189) |
92% (198) |
90% (130) |
91% (138) |
92% (114) |
|
| Oil well logging |
90% (48) |
79% (48) |
86% (42) |
91% (44) |
92% (24) |
|
| Industrial sector |
89% (862) |
84% (916) |
84% (620) |
85% (633) |
79% (483) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Security | Industrial Sector |
94% (828) |
95% (873) |
91% (610) |
94% (624) |
94% (484) |
B.5.3 Academic and research sector
Tables 12 to 15 in this appendix shows the inspection performance of licensees in the Academic and Research sector. The performance of the subsectors is shown for the years 2015 – 2019 as a percentage of the inspections that received fully satisfactory or satisfactory grades for the SCA and the total number of inspections for which performance in that SCA was assessed. The number of inspections for the Academic and Research sector is the aggregate for the entire sector, including subsectors not highlighted in Part I.
A breakdown by subsector is not provided for the security SCA given the potentially sensitive information associated with that SCA.
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Management system | Laboratory studies and consolidated use |
95% (61) |
97% (71) |
97% (73) |
99% (84) |
99% (74) |
| Academic and research sector |
94% (71) |
97% (75) |
97% (73) |
99% (86) |
99% (74) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Operating Performance | Laboratory studies and consolidated use |
75% (63) |
92% (75) |
97% (74) |
88% (86) |
95% (74) |
| Academic and research sector |
78% (77) |
91% (81) |
97% (75) |
88% (90) |
95% (74) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Radiation Protection | Laboratory studies and consolidated use |
75% (63) |
92% (75) |
97% (74) |
88% (86) |
93% (74) |
| Academic and research sector |
78% (77) |
91% (81) |
97% (75) |
88% (90) |
93% (74) |
| SCA | Subsector or sector | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Security | Academic and research sector |
91% (70) |
96% (73) |
96% (69) |
79% (72) |
99% (73) |
B.5.4 Commercial sector
Table 16 shows the inspection performance of licensees in the commercial sector. The performance of the sectors is shown for the years 2015 – 2019 as a percentage of the inspections that received fully satisfactory or satisfactory grades for the SCA and the total number of inspections for which performance in that SCA was assessed. The number of inspections for the commercial sector is the aggregate for the entire sector.
Due to the small number of inspections in each subsector, a breakdown by subsector is not provided. Identifying trends would be difficult in subsectors due to the low number of licensees in many subsectors.
| SCA | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Management System |
94% (117) |
97% (87) |
93% (62) |
97% (41) |
97% (40) |
| Operating Performance |
94% (123) |
92% (91) |
94% (67) |
92% (48) |
89% (36) |
| Radiation Protection |
92% (125) |
92% (90) |
95% (63) |
100% (46) |
83% (48) |
| Security |
97% (97) |
99% (70) |
94% (53) |
93% (41) |
91% (35) |
Performance in the environmental protection SCA and conventional health and safety SCA are reported on only for the waste nuclear substance subsector. No waste nuclear substance licensees received below expectations or unacceptable ratings in the environmental protection SCA. The waste nuclear substance licensees continue to manage and monitor environmental releases as a result of licensed activities. These releases are kept well below regulatory limits. There were no unplanned releases to the environment as a result of licensed activities in 2019.
No waste nuclear substance licensees received below expectations or unacceptable ratings in the conventional health and safety SCA. The licensees continue to implement a health and safety program in accordance with the applicable occupational health and safety legislation to protect the health and safety of their workers.
Appendix C: Enforcement Actions Issued in 2019
In 2019, CNSC staff issued 13 orders and zero AMPs to licensees covered by Part I (Figure 10). Three of the orders are still open. The majority of the enforcement actions were issued to licensees in the industrial sector, consistent with previous years. A list of orders issued are included in table 17.
For one licensee, other enforcement actions were required by the CNSC in addition to an order. The CNSC required a warrant to seize devices from the licensee and a court order to dispose of the seized devices through a licensed disposal provider. This was presented to the commission in CMD 19-H107.
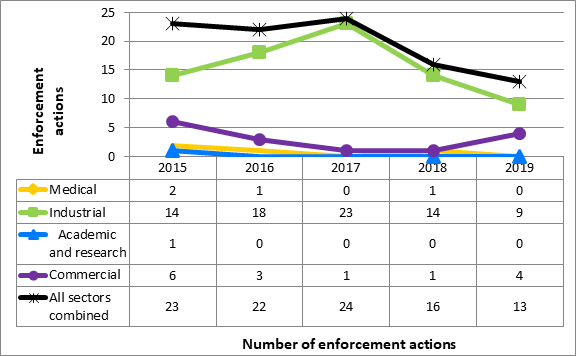
Figure 10: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Medical | 2 | 1 | 0 | 1 | 0 |
| Industrial | 14 | 18 | 23 | 14 | 9 |
| Academic and research | 1 | 0 | 0 | 0 | 0 |
| Commercial | 6 | 3 | 1 | 1 | 4 |
| All sectors combined | 23 | 22 | 24 | 16 | 13 |
|
Issue date (2019) |
Order # | Location | Licensee |
Sector, subsector |
Licensee response |
Closure date (2019) |
|---|---|---|---|---|---|---|
| March 7, 2019 | 1207 | Brampton, Ontario | Orbit Engineering Limited | Industrial Sector, Portable Gauge | Ceased all use of its portable gauges and made immediate provisions to secure and store them until improvements were made to the management oversight of the radiation protection program and all items of non-compliance were corrected to the satisfaction of the CNSC. | March 15 |
| March 20, 2019 | 0838 | St. Catharines, Ontario | Trenergy Inc | Industrial Sector, Industrial Radiography | Immediately placed all radioactive materials in secure storage and ceased all activities involving radioactive materials until improvements were made to the management control of the radiation protection program and work practices to the satisfaction of the CNSC. Corrected all items of non-compliance to the satisfaction of the CNSC. | April 26 |
| March 22, 2019 | N/A | Port Alice, British Columbia | Neucel Specialty Cellulose Ltd. | Industrial Sector, Fixed Gauge | The licensee failed to comply with the order within the specified time frame. The RCMP obtained and then executed a warrant on May 30, 2019 to seize the devices at the request of the CNSC. At this time, CNSC staff accompanied a licensed third party who removed all devices at the location and transported them to a licensed site for secure storage. The Federal Court authorised the CNSC to dispose of the seized gauges on January 29th 2020. On June 25, 2019, the Commission approved the revocation of Neucel’s licence. | The CNSC seized the gauges on May 30, 2019. The Commission revoked the licence on June 25, 2019. |
| April 24, 2019 | 0759 | Calgary, Alberta | Canadian Construction Materials Engineering & Testing Inc | Industrial Sector, Portable Gauge | Ceased all use and transportation of its radiation devices and immediately placed them into secure storage until improvements were made to the management control of the radiation protection program and work practices and all items of non-compliance were corrected to the satisfaction of the CNSC. | May 10 |
| April 25, 2019 | 1117 | Edmonton, Alberta | Alpha Adroit Engineering Ltd | Industrial Sector, Portable Gauge | Ceased all use and transportation of its radiation devices and immediately placed them into secure storage until improvements were made to the radiation protection program and all items of non-compliance were corrected to the satisfaction of the CNSC. | May 7 |
| May 28, 2019 | 0599 | Red Deer, Alberta | Union Street Geotechnical Ltd. | Industrial Sector, Portable Gauge | Prohibited the worker from operating portable gauges until the worker received training in radiation safety and in the safe use of portable gauges to the satisfaction of the CNSC. Corrected all items of non-compliance to the satisfaction of the CNSC. | May 30 |
| June 18, 2019 | 1112 | Long Harbour, Newfoundland | Vale Newfoundland & Labrador Limited | Industrial Sector, Fixed Gauge | Immediately ceased worker entry into vessels fitted with radiation devices until appropriate training was developed for workers entering vessels fitted with radiation devices, a system was developed to ensure training was provided to workers entering such vessels and improved documentation related to vessel entry requirements to the satisfaction of the CNSC. Corrected all non-compliances to the satisfaction of the CNSC. | October 3 |
| July 10, 2019 | 1224 | Wonowon, British Columbia | Allnorth Consultants Limited | Industrial Sector, Portable Gauge | Removed the worker from all activities involving radiation devices until the worker was adequately retrained in all aspects of safe radiation device handling and operations and until all items of non-compliance were corrected to the satisfaction of the CNSC. | September 12 |
| September 12, 2019 | 0561 | Montreal, Québec | Montreal Neurological Institute and Hospital | Commercial, Isotope Production Accelerator Facility | Restricted hotcell activity to ensure that doses to workers are ALARA. The licensee improved the safety of the handling procedures. After implementing the changes, there was a reduction in the extremity doses to employees. | November 5 |
| September 23, 2019 | 1058 | Delta, British Columbia | High Precision Monitoring & Analysis Ltd. | Industrial Sector, X-ray Fluorescence Device | A warrant was planned for early 2020 and had to be put on hold due to COVID-19 concerns with the execution of the warrant. | Order is still open |
| December 6, 2019 | 1209 | Location information will be provided when the order is closed | Licensee information will be provided when the order is closed | Commercial | This order was issued due to security related concerns and remains open. | Order is still open |
| December 13, 2019 | 6062675 | Brantford, Ontario | Mississauga Metal and Alloys Inc. | WNSL | The licensee working on revising the radiation protection program to address the order. Financial impacts of COVID-19 have resulted in reallocation of resources and delayed completion of revisions to the radiation protection program. | Order is still open |
| Dec. 19, 2018 | n/a | Burlington, ON | Isologic Innovative Radiopharmaceuticals Ltd. |
Commercial sector, Processing of nuclear substances |
The Licensee has demonstrated a strong commitment in improving the safety of their I-131 production activities through:
|
January 7, 2020 |
Appendix D: Doses to Workers
A total of 63,015 workers in the four sectors covered in Part 1 were monitored for occupational doses in 2019, 26,539 of whom were classified as Nuclear Energy Workers (NEWs). The differences in doses to workers among sectors reflect the nature of the various activities within those sectors. Figure 11 shows the doses received by the 26,539 NEWs monitored in 2019, while figure 12 shows the doses of NEWs from 2015 to 2019.
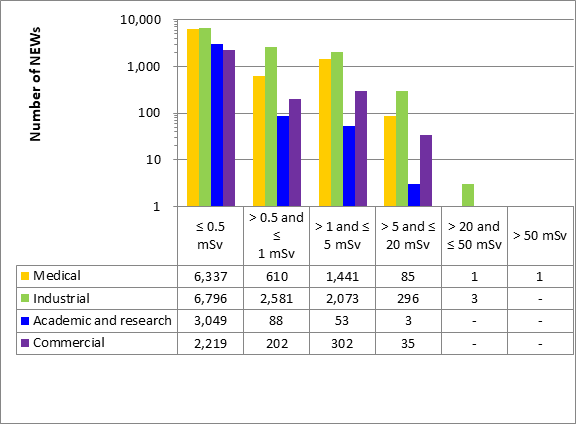
Figure 11: Text version
| ≤ 0.5 mSv | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| Medical | 6,337 | 610 | 1,441 | 85 | 1 | 1 |
| Industrial | 6,796 | 2,581 | 2,073 | 296 | 3 | - |
| Academic and research | 3,049 | 88 | 53 | 3 | - | - |
| Commercial | 2,219 | 202 | 302 | 35 | - | - |
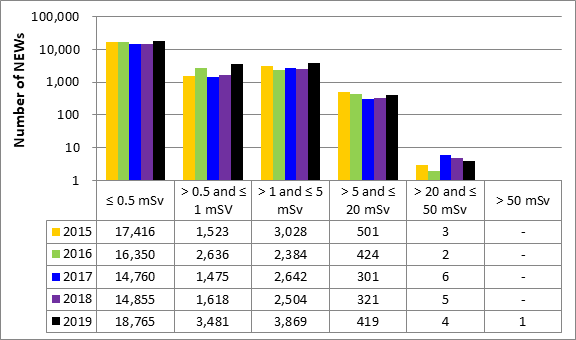
Figure 12: Text version
| ≤ 0.5 mSv | > 0.5 and ≤ 1 mSV | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| 2015 | 17,416 | 1,523 | 3,028 | 501 | 3 | - |
| 2016 | 16,350 | 2,636 | 2,384 | 424 | 2 | - |
| 2017 | 14,760 | 1,475 | 2,642 | 301 | 6 | - |
| 2018 | 14,855 | 1,618 | 2,504 | 321 | 5 | - |
| 2019 | 18,765 | 3,481 | 3,869 | 419 | 4 | 1 |
In 2019, one non-NEW worker in the medical sector received a dose of 1.85 mSv. This is above the regulatory limit of 1 mSv/year for a worker who is not a NEW. The licensee could not come up with a reasonable explanation for the exceedance. The manufacturer did not find any anomalies with the dosimeter. CNSC staff issued a return to work letter with increased monitoring with a direct reading dosimeter. All doses recorded during the 6 month period after the event were normal.
In 2019, a NEW exceeded the whole body dose limit of 50 mSv. The employee was from the medical sector and the dose to the employee was 57 mSv. The licensee's investigation did not reveal any root cause for the dose and the dose is likely non-personal; however a non-personal dose assumption cannot be supported. The worker was initially removed from tasks that could add to the dose. On December 18, 2019, the licensee made a request to the CNSC for an authorization to return the affected worker to work. CNSC staff evaluated this request and the authorization was granted. This event was reported at the Commission hearing in June 2020.
D.1 Medical sector
This appendix shows the doses received by NEWs in the medical sector, as reported to the CNSC in 2019 (Figure 13). Note that the total number of NEWs shown in the medical sector row is the aggregate for the entire sector, including subsectors not highlighted in Part I. Results are similar to past years.
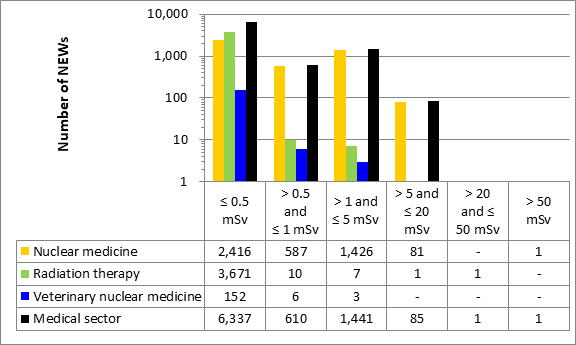
Figure 13: Text version
| ≤ 0.5 mSv |
> 0.5 and ≤ 1 mSv |
> 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| Nuclear medicine | 2,416 | 587 | 1,426 | 81 | - | 1 |
| Radiation therapy | 3,671 | 10 | 7 | 1 | 1 | - |
| Veterinary nuclear medicine | 152 | 6 | 3 | - | - | - |
| Medical sector | 6,337 | 610 | 1,441 | 85 | 1 | 1 |
D.2 Industrial sector
This appendix shows the doses received by NEWs in the industrial sector, as reported to the CNSC in 2019 (Figure 14). Note that the total number of NEWs shown in the industrial sector row is the aggregate for the entire sector, including subsectors not highlighted in Part I. Results are similar to past years.
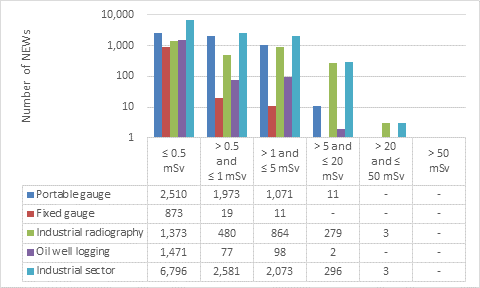
Figure 14: Text version
| ≤ 0.5 mSv |
> 0.5 and ≤ 1 mSv |
> 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| Portable gauge | 2,510 | 1,973 | 1,071 | 11 | - | - |
| Fixed gauge | 873 | 19 | 11 | - | - | - |
| Industrial radiography | 1,373 | 480 | 864 | 279 | 3 | - |
| Oil well logging | 1,471 | 77 | 98 | 2 | - | - |
| Industrial sector | 6,796 | 2,581 | 2,073 | 296 | 3 | - |
D.3 Academic and research sector
This appendix shows the doses received by NEWs in the academic and research sector, as reported to the CNSC in 2019 (Figure 15). Note that the total number of NEWs shown in the academic and research sector row is the aggregate for the entire sector, including subsectors not highlighted in Part I. Results are similar to past years.
Doses received by NEWs working at the CNSC laboratory remained very low, with all workers receiving doses below 0.5 mSv.
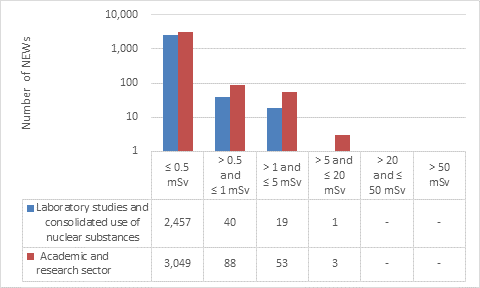
Figure 15: Text version
| ≤ 0.5 mSv |
> 0.5 and ≤ 1 mSv |
> 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
|
Laboratory studies and consolidated use of nuclear substances |
2,457 | 40 | 19 | 1 | - | - |
|
Academic and research sector |
3,049 | 88 | 53 | 3 | - | - |
D.4 Commercial sector
This appendix shows the doses received by NEWs in the commercial sector, as reported to the CNSC in 2019 (Figure 16). Note that the total number of NEWs shown in the commercial sector row is the aggregate for the entire sector, including subsectors not highlighted in Part I. Results are similar to past years.
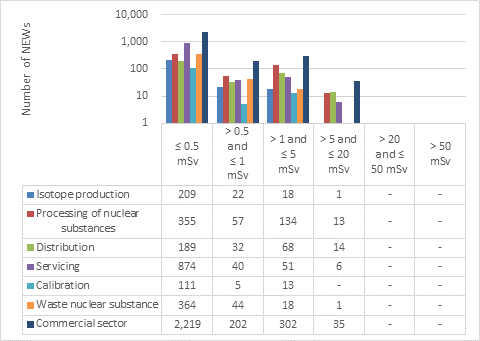
Figure 16: Text version
| ≤ 0.5 mSv |
> 0.5 and ≤ 1 mSv |
> 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| Isotope production | 209 | 22 | 18 | 1 | - | - |
| Processing of nuclear substances | 355 | 57 | 134 | 13 | - | - |
| Distribution | 189 | 32 | 68 | 14 | - | - |
| Servicing | 874 | 40 | 51 | 6 | - | - |
| Calibration | 111 | 5 | 13 | - | - | - |
| Waste nuclear substance | 364 | 44 | 18 | 1 | - | - |
| Commercial sector | 2,219 | 202 | 302 | 35 | - | - |
Appendix E: Reported Events
Licensees are required to have programs in place for the management of unplanned events and accidents. The events that warrant mandatory reporting and the content of the reports are set out in the NSCA, its regulations and the licence conditions. CNSC staff review, assess and track all events reported by licensees.
Since 2014, reported events have been ranked using the International Nuclear and Radiological Event Scale (INES), a tool for communicating the safety significance of nuclear and radiological events to the public. Note that the scale is not a tool for comparing safety performances among facilities or organizations, but rather for effectively communicating the safety significance of events.
In 2019, 183 events related to nuclear substances and prescribed equipment were reported to the CNSC (Figure 17). Of these events, 181 ranked as INES level 0 (no safety significance), one was ranked as INES level 1 (anomaly), and one was ranked as INES level 2 (incident). A description of each event can be found in Table 18.
The INES level 1 event involved non-NEW that exceeded the annual limit of 1 mSv (refer to the event previously discussed in Appendix D Doses to Workers).
The INES level 2 event reported in 2019 involved a NEWs with an annual whole body dose in excess of the regulatory limits (refer to the event previously discussed in Appendix D Doses to Workers).
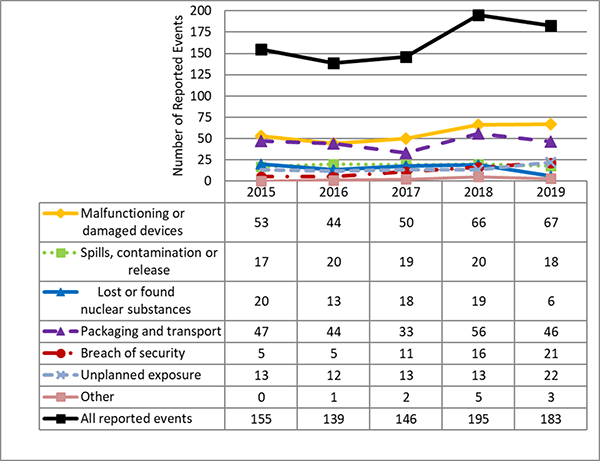
Figure 17: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
|
Malfunctioning or damaged devices |
53 | 44 | 50 | 66 | 67 |
| Spills, contamination or release | 17 | 20 | 19 | 20 | 18 |
|
Lost or found nuclear substances |
20 | 13 | 18 | 19 | 6 |
| Packaging and transport | 47 | 44 | 33 | 56 | 46 |
| Breach of security | 5 | 5 | 11 | 16 | 21 |
| Unplanned exposure | 13 | 12 | 13 | 13 | 22 |
| Other | 0 | 1 | 2 | 5 | 3 |
| All reported events | 155 | 139 | 146 | 195 | 183 |
Note: Unplanned exposures include individuals crossing safety barriers while industrial radiography was occurring, skin contamination events below regulatory limits, any events where procedures were not followed and workers received dose below regulatory limits, and any events where regulatory limits were exceeded.
| # | Date reported | INES rating | Event type | Sector | Event summary |
|---|---|---|---|---|---|
| 3549 | 2019-01-01 | 0 | Breach of security | Industrial | The exterior fence at a facility was cut open and a fuel transfer pump was stolen. No attempt was made to access the radiation storage bunker. Local police were notified. The fence was repaired. The event is closed. |
| 3554 | 2019-01-07 | 0 | Packaging and transport | Commercial | A vehicle carrying fluorine-18 was involved in a motor vehicle collision. There was no damage to the Type A package. The event is closed. |
| 3566 | 2019-01-07 | 0 | Unplanned exposure | Medical | A NEW received skin contamination from technetium-99m while injecting a nuclear medicine patient. The worker was wearing personal protective equipment but received contamination on the face, neck, hair and arms. Decontamination procedures were followed immediately. The CNSC radiation protection staff reviewed the situation and confirmed that the dose received was well within the regulatory limits. This event is considered closed with no further actions required. |
| 3553 | 2019-01-10 | 0 | Packaging and transport | Commercial | A vehicle transporting fluorine-18 was involved in a motor vehicle collision. There was no damage to the Type A package. The event is closed. |
| 3557 | 2019-01-10 | 0 | Breach of security | Medical | A construction worker bypassed the card access and PIN security system by short circuiting the access panel in order to complete work in a restricted area. No sources went missing. The contractor did not receive an overexposure as a result of the incident. Contract staff have been retrained. The event is closed. |
| 3558 | 2019-01-10 | 0 | Lost | Medical | A cobalt-57 (1.85 MBq) was reported lost. The licensee suspects it was sent out with the laundry. This is a very low risk sealed source. There is no risk to the public. The event is closed. |
| 3567 | 2019-01-17 | 0 | Contamination | Academic and Research | A routine leak test performed by the licensee on a nickel-63 sealed source in an electron capture device in storage revealed the presence of contamination >200 Bq. The security of the device has been maintained and there is no impact to the environment or health and safety of persons. The licensee has committed to transferring this device to an appropriate licensed waste facility. The event is considered closed |
| 3564 | 2019-01-18 | 0 | Unplanned exposure | Industrial | A NEW was inside a radiography barrier while the cobalt-60 source was in an unshielded position. The estimated dose to the NEW as a result of the incident was 0.36 mSv. The incident was the result of miscommunication between the two NEWs working at the site. Corrective actions are being implemented to prevent future recurrence. The event is closed. |
| 3570 | 2019-01-20 | 0 | Packaging and transport | Industrial | A Type A package containing a technitum-99m generator (111 GBq) sustained minor damage. The damage had no impact on the integrity of the package. The licensee recommended that the package continue to the destination. Consignee surveyed the package upon receipt and confirmed that all readings and swipes were within normal limits. The event is closed. |
| 3574 | 2019-01-30 | 0 | Unplanned exposure | Medical | A NEW received contamination to their hair and nasal cavity while preparing radiopharmaceuticals in the hot lab. The estimated dose to the NEW was 72.5 mSv to the skin, which is below the regulatory limit of 500 mSv. The event is closed. |
| 4577 | 2019-02-04 | 0 | Device malfunction | Industrial | The shutter on a portable gauge in storage was partially open. Manual attempts to close the shutter were unsuccessful. After the workers cleaned the gauge, they were able to successfully close the shutter. A survey meter confirmed that the radiation levels were at background. The event is closed. |
| 4575 | 2019-02-05 | 0 | Breach of security | Medical | A licensed cancer center experienced a security breach when an individual obtained a set of master keys. The keys did not give access to the areas where radiation source or prescribed equipment are located. All physical barriers to these areas remained in place. Corrective actions have been taken by the license. The event is closed. |
| 4576 | 2019-02-07 | 0 | Device malfunction | Industrial | The radiation readings near the shutter of a fixed gauge were higher than normal. It was determined that the shutter on the fixed gauge was malfunctioning. The fixed gauge was removed from the licensee's possession by a licensed service provider. No overexposures occurred as a result of the incident. The event is closed. |
| 4578 | 2019-02-11 | 0 | Breach of security | Academic and Research | A security system was disarmed when the area was unattended. No one accessed the area during this time and the outer door and the shielding door to the cobalt irradiator were closed and locked. The security system was rearmed as soon as it was discovered. Corrective actions have been implemented by the licensee. This event is considered closed. |
| 4580 | 2019-02-14 | 0 | Spill | Medical | A spill of 2 GBq of fluorine-18 when a vial dropped in a hot lab. No personal skin contamination on the technologist nor surface contamination was detected. Corrective actions have been implemented to prevent future recurrences. No overexposures or personal contamination occurred as a result of this event. The event is closed. |
| 4582 | 2019-02-14 | 0 | Device malfunction | Industrial | The shutter for a fixed gauge would not properly close and the handle appeared to be loose. The activity from the device was normal and work was not being conducted near the device. The device was repaired by a licensed service provider. The event is closed. |
| 4581 | 2019-02-15 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4583 | 2019-02-16 | 0 | Packaging and transport | Commercial | A vehicle transporting empty vials of fluorine-18 FDG was involved in a motor vehicle collision. There was no damage to the package. The event is closed. |
| 4589 | 2019-02-19 | 0 | Spill | Medical | A major spill of indium-111 greater than 100 EQ was reported. The room was cleaned and the area with fixed contamination was covered with shielding material. No personal contamination occurred due to the incident. The incident did not cause any effects to the safety of workers and the environment. The event is closed. |
| 4630 | 2019-02-19 | 0 | Barrier breach | Industrial | A non-NEW crossed a radiography barrier despite verbal warnings from the CEDOs on site. The worst-case scenario estimated dose of 0.00073 mSv to the non-NEW (below the public dose limit of 1 mSv). No overexposures occurred as a result of this. The event is closed. |
| 4587 | 2019-02-21 | 0 | Contamination | Commercial | An empty transport package returned by a client was contaminated with technetium-99m. There was also contamination on the driver’s hands and in the vehicle. The driver's hands were decontaminated below limits and the case was stored for further decay. There were no overexposures as a result of this event. The event is closed. |
| 4590 | 2019-02-24 | 0 | Device malfunction | Industrial | A sealed source used in radiography source got stuck in a shielded position. This was detected during pre-operational checks. The guide tube and device source were both taken out of service. The event is closed. |
| 4593 | 2019-02-24 | 0 | Breach of security | Medical | A licensee had an unplanned power outage which opened a bunker door to a treatment room containing Class II prescribed equipment. The hospital's security department was not notified of the power outage and the bunker door was left open until it was closed by an authorized user. The outer glass door in front of the bunker door remained locked by a physical key. Security procedures have been updated to prevent future recurrences. No overexposures occurred as a result of this event and all sources were accounted for. The event is closed. |
| 4594 | 2019-02-26 | 0 | Device malfunction | Industrial | A sealed source in an exposure device was stuck in an unshielded position when the source became disconnected from the control cable. The licensee successfully performed a source retrieval. The maximum dose to NEWs was 11 uSv. No overexposures occurred as a result of this event. The device has been taken out of service for maintenance. The event is closed. |
| 4597 | 2019-02-28 | 0 | Device malfunction | Industrial | Non-NEWs were performing work around a fixed gauge whose shutter failed to close. The workers did not follow proper procedures and were not using a survey meter. CNSC staff conducted worst case dose estimates and confirmed that no overexposures occurred as a result of this event. The event is closed. |
| WNSL1 | 2019-03-01 | 0 | Packaging and transport | Commercial | Four sea containers received from an international nuclear power plant arrived at the licensee’s facility were not labelled with the proper transport category levels and overpack label. |
| 4599 | 2019-03-03 | 0 | Stolen | Industrial | A radiography truck with an exposure device was stolen from a licensee's location. The incident was reported to the police. Using the onboard GPS tracker, the truck and exposure device were recovered the next day. The event is closed. |
| 4602 | 2019-03-06 | 0 | Contamination | Medical | The licensee reported contamination above regulatory limits. The contamination was cleaned up and the licensee revised their procedures to prevent further incidents. The event is closed. |
| 4604 | 2019-03-06 | 0 | Unplanned exposure | Medical | A student (NEW) received a dose that exceeded the action level but was below regulatory limits. The cause of the elevated dose was determined to be the student’s inexperience, including possible contamination of the dosimeter. The event is considered closed. |
| 4603 | 2019-03-07 | 0 | Unplanned exposure | Medical | A nuclear medicine technologist received a dose to the extremity of 36.6 mSv (below the regulatory limit of 500 mSv) when they knocked a syringe containing technetium-99m. The individual involved was a NEW. The event is closed. |
| 4611 | 2019-03-12 | 0 | Packaging and transport | Commercial | A licensee received a package with minor damage. There was a small puncture in the corner of the package, the courier noticed this and notified the consignee who sent staff to monitor the package. There was no damage to the contents or shielding, and no leaks were detected. The event is closed. |
| 4612 | 2019-03-18 | 0 | Lost | Academic and Research | The licensee reported that a liquid scintillation counter was lost from their inventory. The radiation device contained 592 kBq of cesium-137. Due to the low activity of the source (Category 5), there is on risk to the public or workers. The event is closed. |
| 4613 | 2019-03-20 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4614 | 2019-03-21 | 0 | Device malfunction | Industrial | A portable gauge was discovered in the secure storage room with the shutter open. The RSO immediately closed shutter and the device was sent to the licensed service provider for servicing. There were no overexposures as a result of this incident. The event is closed. |
| 4629 | 2019-03-21 | 0 | Breach of security | Medical | The RSO was doing a routine check after hours and noticed that the treatment room door was open and a key had been left on the control console of a prescribed equipment. He secured the prescribed equipment and the room as per protocol. An investigation determined that the situation was due to a miscommunication between staff on who was responsible to secure the room. No nuclear substances went missing, and prescribed equipment was not damage. The event is closed. |
| 4615 | 2019-03-26 | 0 | Device malfunction | Industrial | A portable gauge was shipped to a service provider with the shutter open. The device was cleaned and the shutter is functioning. There was no overexposure as a result of this incident. The event is closed. |
| 4618 | 2019-03-29 | 0 | Packaging and transport | Commercial | A vehicle transporting packages of technetium-99m was involved in a minor vehicle accident. There was no damage to the packages. The event is closed. |
| WNSL2 | 2019-04-03 | 0 | Other - Injury | Commercial | A lost time accident occurred in November 2018 but was not reported to the CNSC until April 2019. The event is closed. |
| 4619 | 2019-04-02 | 0 | Device malfunction | Industrial | The shutters on two fixed gauges were failing to closed. Initially, the devices remained in operation in a flagged off area. Later in the year, on the manufacturer's recommendation, the devices were replaced. The malfunctioning devices were sent to a licensed service provider for disposal. The event is closed. |
| 4620 | 2019-04-03 | 0 | Breach of security | Commercial | An unauthorized individual gained access to a cyclotron facility. The individual flipped some electrical switches which alerted the facility's security personnel. The individual id not access restricted areas. The event is closed. |
| 4621 | 2019-04-05 | 0 | Device damaged | Industrial | A technician noticed that some of the welding at the bottom of a fixed gauge was cracked. The fixed gauge was locked out and put in secure storage. The event is closed. |
| 4626 | 2019-04-12 | 0 | Other – flood | Medical | The licensee reported a flood in a nuclear medicine facility. The facility was decommissioned and surveyed. The damaged equipment was being replaced. The event is closed. |
| 4627 | 2019-04-15 | 0 | Lost | Medical | The licensee reported that four sealed sources went missing after a flood in a nuclear medicine facility. Two of the sources are below the exemption quantity. The other two are Category 5 cesium-137 sealed sources. The sealed sources were not found. Due to the low activity of the sources, there is no risk to the public. This event is considered closed. |
| 4628 | 2019-04-16 | 0 | Device damaged | Industrial | A worker backed his car into a portable gauge, causing some damage to the device. Radiation surveys taken after the incident were within the normal range. The licensee made arrangements to repair the portable gauge. The event is closed. |
| 4631 | 2019-04-17 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4633 | 2019-04-23 | 0 | Device damaged | Industrial | A licensee discovered cracks on the weld of a fixed gauge. The likely cause was the age of the radiation device; it is over 35 years old. The radiation device will be replaced in 2020. The event is closed. |
| 4635 | 2019-04-24 | 0 | Spill | Medical | A spill of approximately 400 MBq of fluorine-18 occurred in a hot lab. A NEW received minor skin contamination (4 mSv which is below the limit of 500 mSv) to the arms and face. The cart and equipment were placed in storage for decay. The event is closed. |
| 4637 | 2019-04-24 | 0 | Unplanned exposure | Commercial | A licensee detected elevated dose rates, up to 1 mSv/h, coming from a dumpster. Inside the dumpster they found six molybdenum-99 generators outside their shielding. The molybdenum-99 columns were retrieved and placed in a lead-shielded storage at the warehouse. The maximum dose received as a result of this event is 4.4 mSv to a NEW. The event is closed. |
| 4639 | 2019-04-24 | 0 | Spill | Medical | A spill of 780 MBq of technetium-99m spilled on the floor. No personnel were contaminated. The floor area was cleaned and decontaminated. The event is closed. |
| 4641 | 2019-04-26 | 0 | Breach of security | Medical | A patient at a hospital wandered into a secure area. There was no malicious intent on the part of the patient. There was no risk to the patient as the Class II prescribed equipment was turned off. The event is closed. |
| 4643 | 2019-04-29 | 0 | Packaging and transport | Medical | A package containing a cobalt-57 sealed source of 370 MBq was sustained visible damage. The sealed source was leak tested; no leaks were detected. This event is considered closed. |
| 4651 | 2019-04-30 | 0 | Breach of security | Medical | A security guard found the licensee's brachytherapy room unsecured. There was no loss of nuclear substances or damage to prescribed equipment as a result of this event. The event is closed. |
| 4645 | 2019-05-02 | 0 | Device malfunction | Industrial | The shutter on a portable gauge became stuck after the gauge was used. The device was transported back to the licensee's storage location where the RSO was able to close the shutter. The maximum dose rate measured was 1.9 uSv/hr at two meters. The event is closed. |
| 4649 | 2019-05-08 | 0 | Device damaged | Industrial | An iridium-192 source used in an exposure device could not be returned to the shielded position after a routine exposure. After a few attempts the CEDO was able to return the sealed source to the shielded position. A close inspection of the guide tube revealed that there was damage at the threaded end (near the swage fitting) that had not been visible during the pre-operational checks. The maximum dose to the NEW as a result of this event was 17 uSv, with no doses to members of the public. This event is considered closed. |
| 4653 | 2019-05-08 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4654 | 2019-05-13 | 1 | Unplanned exposure | Medical | A non-NEW received a dose of 1.85 mSv, which exceeds the regulatory limit of 1 mSv. The employee could not come up with a reasonable explanation for the exceedance. The manufacturer did not find any anomalies with the dosimeter. CNSC staff issued a return to work letter with increased monitoring with a direct reading dosimeter. This event is considered closed. |
| 4836 | 2019-05-13 | 0 | Packaging and transport | Commercial | A vehicle transporting one empty package and one Type A package with 58 GBq of fluorine-18 was involved in motor vehicle collision. The packages were not damaged. The driver was transported to the hospital and was released shortly thereafter. The event is closed. |
| 4662 | 2019-05-15 | 0 | Spill | Medical | A technologist dropped a vial of technetium-99m (1,852 MBq). The technologist's shoes and apron were contaminated and placed in decay storage. The maximum whole body dose was estimated to be 2 mSv, which is below regulatory limits. The event is closed. |
| 4657 | 2019-05-19 | 0 | Device malfunction | Industrial | A locking pin was missing from the shutter handle of a fixed gauge, indicating degradation of the radiation device. The RSO was able to close the shutter and survey results were within normal levels. The device was dismounted and placed in storage, until it could be serviced. The event is closed. |
| 4658 | 2019-05-21 | 0 | Unplanned exposure | Medical | A NEW received contamination to the skin from technetium-99m while injecting a nuclear medicine patient. Decontamination procedures were followed immediately. The dose to the skin of the NEW was 8.2 mSv and effective dose to the worker's hand as 0.04 uSv, which is below regulatory limits. The event is closed. |
| WNSL3 | 2019-05-22 | 0 | Packaging and transport | Commercial | The licensee used a damaged container for a shipment. The licensee did not detect the damage during their pre-shipment inspection. There was no radiological release. The event is closed. |
| 4659 | 2019-05-24 | 0 | Device damaged | Industrial | A sealed source used for industrial radiography became stuck in the guide tube when the guide tube was dented. The sealed source could not be retracted into the exposure device. A source retrieval procedure was conducted successfully. The doses to the EDOs (all NEWs) were 380 uSv, 170 uSv and 208 uSv. The event is closed. |
| 4660 | 2019-05-24 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4661 | 2019-05-24 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4665 | 2019-05-29 | 0 | Unplanned exposure | Commercial | A NEW received skin contamination to the hand from technetium-99m. The likely cause was that the worker handled contaminated personal protective equipment (gloves and sleeves) with bare hands. The estimated dose to the NEW's right hand was 50.7 mSv, so below the regulatory limit of 500 mSv. The event is closed. |
| 4674 | 2019-06-06 | 0 | Packaging and transport | Commercial | A vehicle transporting excepted packages was involved in a motor vehicle collision. There was no damage to the packages. The event is closed. |
| 4675 | 2019-06-07 | 0 | Breach of security | Medical | The motion sensor alarm for an irradiator suite was found unarmed. It was re-armed upon discovery. There was no loss of nuclear substances or damage to prescribed equipment as a result. The event is closed. |
| 4693 | 2019-06-07 | 0 | Unplanned exposure | Medical | Two NEWs were contaminated with technetium-99m while performing a perfusion lung study. The syringe was not securely attached to the intravenous lock, which resulted in the dose being ejected outside of the lock and into the air. The two NEWs were contaminated, cleanup procedures were executed. The maximum doses received as a result of this event were 191 mSv to the extremity of the first NEW and 12 mSv whole body dose to the other NEW. These are below the regulatory limits of 500 mSv/year for extremities and 50 mSv/year for the whole body). The event is closed. |
| 4678 | 2019-06-10 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4682 | 2019-06-10 | 0 | Device malfunction | Industrial | A sealed source (iridium-192, 555 GBq) used for industrial radiography could not be retracted into the shielded position in the exposure device due to a loop in the guide tube extension. The exposure device was moved to remove the loop and the sealed source was retracted to the shielded position. The doses received by the NEWs involved in the retrieval were 0.4 mSv and 0.05 mSv (so below regulatory limits but in exceedance of action levels). The event is closed. |
| 4691 | 2019-06-10 | 0 | Barrier breach | Medical | Two window cleaners accessed the roof above the linacs with a ladder. The area has a slightly elevated dose rate (~10 uSv/h) and is supposed to be accessed only with authorization from the physics department. There was no overexposure as a result of this event. The event is closed. |
| 4677 | 2019-06-11 | 0 | Packaging and transport | Commercial | A vehicle transporting packages of technetium-99m was involved in a minor vehicle accident. There was no damage to the packages. The event is closed. |
| 4681 | 2019-06-11 | 0 | Device malfunction | Industrial | A licensee transported a portable gauge with an open shutter to a licensed service provider. The service provider fixed the problem with the shutter. There were no overexposures as a result of this event. This event is considered closed. |
| 4684 | 2019-06-17 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4669 | 2019-06-19 | 0 | Spill | Commercial | A spill of gallium-68 (56 MBq) occurred during the preparation of samples. The NEW involved received a dose to the hand of 15.05 mSv and an effective dose of 0.7 mSv, which is below regulatory limits. The event is closed. |
| 4685 | 2019-06-20 | 0 | Device damaged | Industrial | A licensee reported a damaged portable gauge. There was a crack in the plastic casing likely caused by over-tightened screws. Leak tests were conducted; no leaks were detected. The device was sent for repair by a licensed service provider. The event is closed. |
| 4688 | 2019-06-25 | 0 | Packaging and transport | Commercial | A package was damaged during shipment when it was exposed to rain. Only the external package was damaged, none of the internal packages or contents were damaged. The box was repackaged before being shipped to its final destination. The event is closed. |
| 4694 | 2019-06-27 | 0 | Breach of security | Industrial | There was an attempted break-in at licensee location. A hole was cut in perimeter fence. The licensee was notified by security firm, who secured the area until the RCMP arrived; an inspection of the yard and facility was conducted, and there was no breach of the radiation storage area. The event is closed. |
| 4695 | 2019-06-27 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4697 | 2019-06-28 | 0 | Breach of security | Industrial | A local police force reported that a door at a licensee location with radiation warning signs was open. The licensee determined that the door was not properly locked by the last person exiting the shop. The radiation device was off site at the time of the incident. The event is closed. |
| 4696 | 2019-07-02 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4700 | 2019-07-02 | 0 | Breach of security | Industrial | A licensee's facility was broken into multiple times over a six-day period. The perimeter gate opening mechanism was damaged to gain access to the facility. There was no attempt to access the area where the fixed gauge is stored. RCMP are investigating the incident. The event is closed. |
| 4701 | 2019-07-04 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4705 | 2019-07-04 | 0 | Other | Commercial | A release of carbon-11 occurred from a cyclotron facility. There were no overexposures as a result of this event. The dose to the most vulnerable member of the public and to a NEW at the facility were determined to be very low, less than 0.1 uSv for both cases. This is well below regulatory limits of 1 mSv/year for members of the public and 50 mSv/year for NEWs. The event is closed. |
| 4699 | 2019-07-05 | 0 | Breach of security | Industrial | An unauthorized individual jumped the fence to enter the yard of a licensee's facility. Two personal vehicles were broken into at the facility, but there was no attempt to break in to the radiation storage area onsite, and all equipment and sources were accounted for. The incident was reported to the local police. The event is closed. |
| WNSL4 | 2019-07-05 | 0 | Packaging and transport | Commercial | The licensee used a damaged container for a shipment. The licensee did not detect the damage during their pre-shipment inspection. There was no radiological release. The event is closed. |
| 4704 | 2019-07-08 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4706 | 2019-07-11 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. There was significant damage to the gauge. Leak test were conducted; no leaks were detected. The event is closed. |
| 4710 | 2019-07-11 | 0 | Packaging and transport | Commercial | A vehicle transporting empty packages was involved in a motor vehicle collision. There was no damage to the packages. The event is closed. |
| 4707 | 2019-07-12 | 0 | Device damaged | Industrial | The guide tube of an exposure device was damaged when a heavy piece of equipment fell on it from a height of 1 m. The CEDOs were able to retract the source back into the exposure device. There was no overexposure as a result of this event. The event is closed. |
| 4839 | 2019-07-12 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tests were performed; no leaks were detected. The event is closed. |
| 4711 | 2019-07-17 | 0 | Spill | Commercial | A vial of fluorine-18 (320 MBq) was spilled in a clean room. There was no personal contamination as a result of the incident. Contaminated clothing was set aside for decay. The clean room was closed until radiation levels dropped. The event is closed. |
| 4714 | 2019-07-18 | 0 | Device damaged | Industrial | A licensee shipped a damaged portable gauge to a licensed service provider without notifying the recipient. The damage to the gauge was severe, however the dose rates were within the normal range. Leak tests were conducted; no leaks were detected. The gauge was disposed of. The event is closed. |
| 4716 | 2019-07-19 | 0 | Packaging and transport | Commercial | A vehicle transporting an empty package was involved in a motor vehicle collision. There was no damage to the packages. The event is closed. |
| 4717 | 2019-07-19 | 0 | Breach of security | Industrial | An exposure device was left on a radiography truck without the proper security measures in place. Neither the truck, the storage cabinet for the exposure device, nor the exposure device storage vault were properly locked. No radiation devices were missing as a result of the incident. The event is closed. |
| 4719 | 2019-07-23 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was extensive but the sources stayed in the shielded positions. Leak tests were performed; no leaks were detected. The event is closed. |
| 4721 | 2019-07-25 | 0 | Spill | Medical | A spill of 150 MBq fluorine-18 occurred as a result of a defective infusion kit. There was no skin contamination of either the worker or the patient. The room was decontaminated prior to being used again. The event is closed. |
| 4722 | 2019-07-25 | 0 | Packaging and transport | Medical | A licensee received a damaged package containing a vial of iodine-123. The package was damaged during transport. No contamination was detected on the package and the vial was undamaged. The licensee notified the consignor of the incident. The event is considered closed |
| 4733 | 2019-07-31 | 0 | Packaging and transport | Industrial | A driver of a truck containing well logging tools hit a bunker door at a storage location causing some damage to the door. There was no damage to the sealed sources on board the vehicle, nor to the sealed sources in storage. The damaged infrastructure was repaired. The event is closed. |
| 4734 | 2019-08-02 | 0 | Unplanned exposure | Industrial | A CEDO was exposed accidentally while conducting industrial radiography when the sealed source was exposed prematurely by his working partner due to lack of communication. The maximum dose received as a result of this event was 0.14 mSv. This is below regulatory limits. The event is closed. |
| 4735 | 2019-08-03 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4736 | 2019-08-05 | 0 | Device damaged | Industrial | A licensee discovered cracking around the cap on one of their fixed gauges. Measured dose rates were in the normal range. Leak tests were conducted; no leaks were detected. A licensed service provider and the gauge manufacturer will conduct an investigation to determine the cause of the cracking. The event is closed. |
| 4738 | 2019-08-07 | 0 | Breach of security | Medical | The security alarm on a gamma knife suite (Class II prescribed equipment) was disarmed overnight. This was the second such incident in three months. The event is closed. |
| 4739 | 2019-08-08 | 0 | Breach of security | Industrial | A CEDO and trainee left an exposure device at a client's site. The CEDO returned to get the exposure device when the client informed the licensee that they found the exposure device onsite. There was no exposure to any person as a result of this event. The event is closed. |
| 4605 | 2019-08-16 | 0 | Lost | Medical | An iodine-125 seed (6 MBq; Category 5 sealed source) was missing from storage. The licensee searched for the seed but were unable to find it. Due to the low activity of the source, there is no risk to the public or to workers. The event is closed. |
| 4743 | 2019-08-19 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4744 | 2019-08-23 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was severe. Leak tested were conducted; no leaks were detected. The event is closed. |
| 4745 | 2019-08-23 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was severe. Leak tested were conducted; no leaks were detected. The event is closed. |
| 4746 | 2019-08-27 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tested were conducted; no leaks were detected. The event is closed. |
| 4748 | 2019-08-30 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was severe. Leak tested were conducted; no leaks were detected. The event is closed. |
| 4731 | 2019-09-02 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a motor vehicle collision. The Type A package containing the gauge was ejected on to the road. The Type A package was damaged. The portable gauge also sustained minor damage. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4750 | 2019-09-03 | 0 | Packaging and transport | Commercial | A vehicle transporting excepted packages was involved in a motor vehicle collision. There was no damage to the packages. The event is closed. |
| 4757 | 2019-09-04 | 0 | Device damaged | Industrial | An exposure device failed the No-Go test. The licensee's investigation revealed that the exposure device was dropped by CEDO and damaged. There were no overexposures as a result of this incident. The event is closed. |
| 4751 | 2019-09-05 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4752 | 2019-09-06 | 0 | Packaging and transport | Industrial | A portable gauge fell off of a truck during transport, and was recovered immediately. The worker did not follow internal procedures for securing a portable gauge in the back of the truck. There was no visible damage to the transport gauge or case. The portable gauge was returned to the main storage location and removed from service until leak tests confirmed there were no leaks. The event is closed. |
| 4753 | 2019-09-06 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was stuck in the open position. Dose rates were within the normal range. A licensed service provider removed and disposed of the device. The event is closed. |
| 4754 | 2019-09-06 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tests were performed; no leaks were detected. The event is closed. |
| 4869 | 2019-09-07 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4755 | 2019-09-09 | 0 | Packaging and transport | Industrial | A portable gauge fell from the tailgate of a truck during transport. The authorized user recovered the portable gauge within 15 min. There was no visible damage to the Type A package or the portable gauge. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4807 | 2019-09-12 | 0 | Spill | Commercial | A spill of 100 - 200 MBq gallium-68 occurred in a laboratory when the bottle of containing the gallium-68 was pierced by a needle. There was no skin contamination, overexposure, or release to the environment. The event is closed. |
| 4759 | 2019-09-13 | 0 | Device damaged | Industrial | The plastic casing on a portable gauge was damaged when an authorized user dropped it. Radiation surveys returned dose rates within the normal range. Leak tests were conducted; no leaks were detected. The gauge was sent to a licensed service provider for repair. The event is closed. |
| 4760 | 2019-09-13 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was significant. Leak tests were conducted; no leaks were detected. The portable gauge was repaired by a licensed service provider. The event is considered closed |
| 4762 | 2019-09-16 | 0 | Packaging and transport | Industrial | A vehicle transporting an exposure device was involved in a motor vehicle collision. There was no damage to the exposure device. The event is closed. |
| 4763 | 2019-09-18 | 0 | Spill | Commercial | A spill of 4.588 GBq of technetium-99m occurred in a fume hood. There was no overexposure and no releases to the environment. This event is considered closed. |
| 4764 | 2019-09-18 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge only closed halfway. A licensed service provider repaired the shutter. The event is closed. |
| 4765 | 2019-09-18 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4766 | 2019-09-23 | 0 | Device malfunction | Commercial | During preventative maintenance, a licensee determined that servicing done 11 months before resulted in higher than expected dose rates as a result of an improperly aligned and collimated industrial linear accelerator. There were no unplanned exposures as a result of the incident since the accelerator and resultant beam were in unoccupied areas. |
| 4768 | 2019-09-23 | 0 | Device damaged | Industrial | An exposure device was functioning in an unusual way. The CEDO reported that retracting the source felt unusual. The exposure device and guide tube were taken out of service. The problem was traced to a loose piece of metal from inside the guide tube was affecting the device's function. The event is closed. |
| 4770 | 2019-09-23 | 0 | Breach of security | Academic and Research | The licensee's security system was unarmed overnight. There was no indication of any forced entry. No sealed sources or radiation devices were missing. The event is closed. |
| 4769 | 2019-09-24 | 0 | Barrier breach | Industrial | Three members of the public (client employees) drove into an area where industrial radiography was taking place. The truck drove past radiation warning signs. The CEDO retracted the sealed source quickly. The three individuals in the truck received a dose of 30 uSv. This is below the regulatory limit of 1 mSv/year. The event is closed. |
| 4772 | 2019-09-27 | 0 | Device malfunction | Industrial | An authorized user discovered a portable gauge with a partially open shutter in a licensee's storage location. The portable gauge was cleaned and the shutter functions properly. The event is closed. |
| 4773 | 2019-10-01 | 0 | Packaging and transport | Commercial | A licensee received a package with a broken tie-wrap. The tie-wrap snapped in the delivery truck. The event is closed. |
| 4775 | 2019-10-02 | 0 | Device damaged | Industrial | The shutter on a fixed gauge was stuck in the open position. A licensed service provider removed the gauge and arranged for its disposal. The cause of the stuck shutter was determined to be water entering the device and undergoing repeated freeze-thaw cycles. The event is closed. |
| 4776 | 2019-10-03 | 0 | Spill | Commercial | A vial of technetium-99m was dropped on the floor causing a spill of 25 GBq. The spill area was decontaminated and covered with lead shielding until dose rate measurements returned to normal levels. One worker had contamination on their pants; these clothes were bagged and left to decay. The individual did not have any skin contamination. The event is closed. |
| 4774 | 2019-10-06 | 0 | Device damaged | Industrial | The remote controls for an exposure device were damaged when they came into contact with a hot pipe, which prevented the CEDO from retracting the sealed source back into the device. The sealed source was stuck in an unshielded position. A successful source retrieval was performed, with minimal exposure to the CEDOs involved. The event is closed. |
|
4778 (WNSL5) |
2019-10-08 | 0 | Packaging and transport | Commercial | A truck transporting waste nuclear substances was involved in a motor vehicle collision. There was no damage to the packages and no injuries to the driver. The event is closed. |
| 4777 | 2019-10-08 | 0 | Breach of security | Industrial | On two consecutive nights, unauthorized persons entered the yard of a licensed location by cutting a hole in the fence. Company trucks were vandalized. On the second night, the door to the radiation building was damaged. The individuals did not gain access to the radiation building. The alarm system was still armed and all prescribe event is considered closed. |
| 4779 | 2019-10-10 | 0 | Unplanned exposure | Academic and Research | A NEW tested positive for thyroid uptake of I-124. The uptake to the thyroid gland was 131.16 mSv and committed effective dose of 4.06 mSv. There was no contamination found in the laboratory. All other workers who were working in the laboratory at the same time were also tested; none yielded a positive result for thyroid uptake of iodine. The event is closed. |
| 4783 | 2019-10-10 | 0 | Packaging and transport | commercial | A vehicle transporting packages containing technetium-99m was involved in a motor vehicle collision. The packages were not damaged. The driver was injured and taken to hospital. The event is closed. |
| 4806 | 2019-10-10 | 0 | Device damaged | Industrial | The mounting fixture for a detector on a fixed gauge was damaged. The licensee conducted radiation survey; levels were within usual ranges. The mounting fixture was fixed. The event is closed. |
| 4780 | 2019-10-11 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4782 | 2019-10-12 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was severe. The portable gauge was sent to a licensed service provider for disposal. Leak tested were conducted; no leaks were detected. The event is closed. |
| 4781 | 2019-10-14 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. The source rod was extended at the time. The authorized user was able to retract the source to the shielded position. Radiation surveys were in the normal range. Leak tests were conducted; no leaks were detected. The event is considered closed. |
| 4784 | 2019-10-16 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4785 | 2019-10-16 | 0 | Device malfunction | Industrial | A cobalt-60 sealed source used for industrial radiography became disconnected. The sealed source was transferred to another exposure device, reconnect, and returned to the original device using a new guide tube. The highest dose to a CEDO as a result of this event was 1.10 mSv. The event is closed. |
| 4789 | 2019-10-16 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was malfunctioning. Initially, the shutter would not close when prompted but it did eventually close. The fixed gauge was locked out. The event is closed. |
| 4794 | 2019-10-18 | 0 | Unplanned exposure | Industrial | Two non-NEWs entered a confined space that had a fixed gauge whose shutter was in the open position. No overexposures occurred as a result of the event; the maximum dose received as a result of the event was 4 uSv. The event is closed. |
| 4886 | 2019-10-18 | 0 | Device malfunction | Industrial | The shutter on a portable gauge was stuck in the partially open position. The gauge was packed in its Type A package, locked and tagged out of service. Dose rates measured indicated a small beam with elevated reading. The worker returned the gauge to the storage location. A licensed service provider was able to repair the gauge. The event is closed. |
| 4791 | 2019-10-22 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was malfunctioning and stuck in the open position. The area was cordoned off. The shutter arm of the device was repaired by the manufacturer. The cause of the incident was attributed to metal failure. The event is closed. |
| 4790 | 2019-10-23 | 0 | Spill | Commercial | A spill of 33 GBq iodine-131 occurred in a shielded production box at a licensee's facility. The door to the box was open for operational reasons at the time of the spill. Affected workers underwent thyroid screening. The maximum extremity dose as a result of this event is 3.7 mSv to a NEW (well below limit of 500 mSv). The box was decontaminated and returned to use. The event is closed. |
| 4792 | 2019-10-24 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was minor. The event is closed. |
| 4796 | 2019-10-25 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4797 | 2019-10-25 | 0 | Unplanned exposure | Industrial | A non-NEW was inside barrier during radiography operations. The calculated dose for this non-NEW is 0.006 mSv, which is well below the regulatory limits. The event is closed. |
| 4798 | 2019-10-28 | 0 | Device damaged | Industrial | During annual leak testing a fixed gauge, a licensee discovered a heavily corroded fixed gauge. The radiation surveys taken at the gauge were within the normal levels. Leak tests were performed; no leaks were detected. A licensed service provider removed and disposed of the sealed source inside the gauge so that the fixed gauge could be removed and disposed. The event is closed. |
| 4800 | 2019-10-29 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge would not close. All maintenance activities in the vicinity of the gauge were suspended until a licensed servicing company repaired the shutter on the fixed gauge. The event is closed. |
| 4802 | 2019-11-01 | 0 | Barrier breach | Industrial | Two non-NEWs were working inside a vessel near where industrial radiography was taking place. Based on a recreation of the scenario, the non-NEWs did not receive a dose from the industrial radiography operations. The event is closed. |
| 4803 | 2019-11-04 | 0 | Breach of security | Industrial | Two CEDOs left an exposure device at a client's facility overnight, in an unsecured manner. The exposure device was found the next day at the same place where it had been left. The event is closed. |
| 4804 | 2019-11-05 | 0 | Device damaged | Industrial | The steel rod on a portable gauge was damaged when the worker tried to force the lock. The sealed source remained in its shielded position and a radiation survey confirmed that the dose rates around the gauge were within normal levels. A leak test was conducted, with no leaks detected. The event is closed. |
| 4805 | 2019-11-05 | 0 | Spill | Medical | A spill of 10 GBq of technetium-99m occurred in a laboratory. The vial containing the technetium-99m fell on the floor. The NEWs shoes and pants were contaminated, but there was no skin contamination. The contaminated items were placed in decay storage, the spill was contained with absorbent pads and the laboratory was closed to allow for decay. There were no overexposures as a result of this event. The event is closed. |
| 4811 | 2019-11-12 | 0 | Unplanned exposure | Industrial | A non-NEW crossed the radiography barrier while industrial radiography was taking place. The CEDO immediately retracted the sealed source. The dose rate where the non-NEW was located was 20 uSv/h, and the dose received by the individual was below regulatory limits. The event is closed. |
| 4812 | 2019-11-12 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge in its Type A package slid into a ditch. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4813 | 2019-11-14 | 0 | Breach of security | Academic and Research | A licensee' network drive was hit with a ransomware attack. The drive is shared by laboratory members for common files. The licensee has declined to pay the ransom. The lab was unaware of vulnerability of data on this particular drive. The licensee's network security team is investigating to assess the full extent of the data stolen and how the attack was launched. The event is closed. |
| 4814 | 2019-11-18 | 0 | Lost | Industrial | A Category 5 polonium-210 sealed source (18.5 MBq) was lost by a licensee. The licensee was conducting an inventory check and could not locate the source. The licensee suspects that the sealed source has inadvertently been disposed of as regular waste. Due to the low activity of the sealed source, there is no danger to the public or to the environment. The event is closed. |
| 4819 | 2019-11-21 | 0 | Device damaged | Industrial | A worker using a scissor lift came into contact with the detector section of a fixed gauge. The brackets holding the detector broke and the detector fell. This happened while the shutter was opened. The shutter was closed within a few minutes and the radiation device was locked out of service. The maximum dose received by workers in the vicinity was 4 uSv, so well below the regulatory limit for a member of the public. The detector was repaired and the fixed gauge was returned to service. The event is closed. |
| 4820 | 2019-11-26 | 0 | Device damaged | Industrial | A portable gauge was damaged at a construction site. The damage was significant. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4822 | 2019-11-27 | 2 | Unplanned exposure | Medical | A NEW's dose report for a three-month wearing period showed a reading of 57 mSv, which is above the regulatory limit of 50 mSv. The licensee's investigation did not reveal any root cause for the dose but a non-personal dose assumption cannot be supported. The event is closed. |
| 4821 | 2019-11-29 | 0 | Unplanned exposure | Academic and Research | Five NEWs were contaminated with lutetium-177 while working in a hot lab. The maximum skin dose received to the extremity was estimated at 121 mSv, so below the regulatory limit of 500 mSv. There was no contamination outside of the hot lab and no threat to the public or environment as a result of this event. The event is closed. |
| 4825 | 2019-12-02 | 0 | Spill | Academic and Research | A licensee's employee entered a storage area for a routine inventory verification. Their survey instrument indicated higher than normal tritium air concentration. The worker left the room immediately and had a bioassay that indicated a preliminary dose estimate received of approximately 0.22 mSv. It was determined that two tritium gas cylinders were leaking in the storage room. The licensee determined that 113 GBq of H-3 were released. The leaking cylinders were removed to a fume hood with monitored exhaust until disposal. The event is closed. |
| 4824 | 2019-12-03 | 0 | Device damaged | industrial | A portable gauge was damaged at a construction site. The damage was substantial. Leak tests were conducted; no leaks were detected. The event is closed. |
| 4828 | 2019-12-03 | 0 | Device damaged | industrial | A portable gauge was damaged when it fell off the back of a truck. The authorized user was driving from one compaction testing location to their next work location and received a call on the radio that their gauge had fallen out of the back of their truck. The tailgate in the back of the truck had been left open. The plastic housing on the gauge was cracked. The event is closed. |
| 4830 | 2019-12-05 | 0 | Unplanned exposure | industrial | Two non-NEWs performed a leak test on a fixed gauge while the shutter was open. This was contrary to the licensee's procedure. The maximum dose estimates for the workers involved was 4 uSv, which is below regulatory limits. The event is closed. |
| 4832 | 2019-12-06 | 0 | Device damaged | industrial | A portable gauge sustained minor damage to the casing when the gauge was buried after the wall of a trench collapsed. Emergency procedures where initiated by the worker and the RSO contacted. Dose rate measurements confirmed the sources were in the shielded position. The event is closed. |
| 4833 | 2019-12-06 | 0 | Device damaged | industrial | The casing on a portable gauge was damaged after a worker dropped the portable gauge from a height of 0.5 m. Does rate measurements were at normal levels. The portable gauge was transferred to a licensed service provider for repair. The event is closed. |
| 4834 | 2019-12-06 | 0 | Device damaged | industrial | There was a fire in a building where four fixed gauges were located. Each gauge contains five Cs-137 sources. The fire was extinguished a day after it began. After the fire was extinguished, a survey around the building measured only background radiation readings. All personnel entering the area were required to use electronic dosimeters, which also showed only background radiation levels. The area where the gauges were located was cleared for access 14 days after the fire was extinguished. A technician from a licensed service provider determined that the gauges were compromised; some lead melted and escaped. Radiation levels were measured approximately 60 uSv/hr at the gauge. The gauges will be removed by a licensed service provider who will arrange for their disposal. The event is closed. |
| 4835 | 2019-12-06 | 0 | Spill | Academic and Research | A major spill of fluorine-18 spill in a fume hood and on to the floor of a laboratory. There was no personal contamination as a result of the incident. The laboratory was closed to allow for decay. The event is closed. |
| 4837 | 2019-12-09 | 0 | Device malfunction | industrial | A portable gauge was transported with the shutter open to a licensed service provider. The service provider cleaned the shutter of debris and the device is functioning normally. The event is closed. |
| 4840 | 2019-12-12 | 0 | Device damaged | industrial | A portable gauge was damaged at a construction site. The damage was minor. Leak tests were performed; no leaks were detected. The event is closed. |
| 4848 | 2019-12-16 | 0 | Device malfunction | industrial | Two portable gauges were sent to a licensed service provider were shipped with the shutter open. There was a build-up of material on the gauges preventing the shutters from closing properly. The gauges were cleaned and the shutters were closed successfully by the licensed service provider. The event is closed. |
| 4843 | 2019-12-17 | 0 | Device damaged | industrial | An exposure device was damaged when it was dropped from a height of 12 m. The device has been repaired and returned to use. The incident occurred when the rope being used to hoist the device broke. The event is closed. |
| 4844 | 2019-12-17 | 0 | Device damaged | industrial | A portable gauge was damaged when it fell off a bridge. The damage was minor. Radiation survey measurements within the normal range. The event is closed. |
| 4847 | 2019-12-18 | 0 | Device malfunction | industrial | A portable gauge had a stuck shutter. The shutter was cleaned and the shutter is now operating normally. The event is closed. |
| 4851 | 2019-12-19 | 0 | Packaging and transport | industrial | A vehicle transporting a portable gauge in its Type A package was involved in a motor vehicle collision. There was no damage to the Type A package or to the portable gauge. The event is closed. |
| 4850 | 2019-12-20 | 0 | Device malfunction | Industrial | A licensed service provider received two portable gauges with open shutters on the same afternoon. The gauges were from different licensees. The devices were serviced and the shutters operated properly. The event is closed. |
Appendix F: Inspections conducted in 2019
| Inspection date | Licensee name | City | Province | Inspection type | Sector |
|---|---|---|---|---|---|
| 2019-01-03 | IRISNDT Corp. | Calgary | AB | Type II | industrial |
| 2019-01-03 | Global Engineering & Testing Ltd | Calgary | AB | Type II | industrial |
| 2019-01-03 | Terracon Geotechnique Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-03 | Stelco Inc. | Hamilton | ON | Type II | industrial |
| 2019-01-04 | Mistras Canada, Inc. | Calgary | AB | Type II | industrial |
| 2019-01-07 | Gemtec Consulting Engineers and Scientists Limited | Halifax | NS | Type II | industrial |
| 2019-01-08 | Custom Fabricators & Machinists Limited | Dartmouth | NS | Type II | industrial |
| 2019-01-08 | Stantec Consulting Ltd. | Dartmouth | NS | Type II | industrial |
| 2019-01-08 | CRH Canada Inc. | King City | ON | Type II | industrial |
| 2019-01-08 | Fisher Environmental Ltd. | Markham | ON | Type II | industrial |
| 2019-01-09 | E.F. Monk Holdings Limited | Dartmouth | NS | Type II | industrial |
| 2019-01-09 | Orbit Engineering Limited | Brampton | ON | Type II | industrial |
| 2019-01-09 | Intertape Polymer Inc. | Truro | NS | Type II | industrial |
| 2019-01-10 | Atlas Testing Labs & Services (Nova Scotia) Ltd. | Salt Springs | NS | Type II | industrial |
| 2019-01-14 | Thurber Engineering Ltd. | Oakville | ON | Type II | industrial |
| 2019-01-14 | WorleyParsons Canada Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-14 | WorleyParsons Canada Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-14 | PanPacific Wireline Services Inc. | Brampton | ON | Type II | industrial |
| 2019-01-14 | Roke Technologies Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-15 | Aurora Inspection Limited | Olds | AB | Type II | industrial |
| 2019-01-15 | Stanley Inspection Canada Ltd. | Olds | AB | Type II | industrial |
| 2019-01-16 | Gamma-Tech Inspection Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-16 | Domtar Inc. | Espanola | ON | Type II | industrial |
| 2019-01-16 | Cordax Evaluation Technologies Inc. | Calgary | AB | Type II | industrial |
| 2019-01-17 | Vale Canada Limited | Levack | ON | Type II | industrial |
| 2019-01-17 | Vale Canada Limited | Copper Cliff | ON | Type II | industrial |
| 2019-01-17 | Vale Canada Limited | Lively | ON | Type II | industrial |
| 2019-01-17 | Englobe Corp. | London | ON | Type II | industrial |
| 2019-01-17 | Spectrum NDT Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-17 | Integrated Sustainability Consultants Ltd. | Calgary | AB | Type II | industrial |
| 2019-01-17 | LDS Consultants Inc. | London | ON | Type II | industrial |
| 2019-01-17 | MTE Consultants Inc. | Stratford | ON | Type II | industrial |
| 2019-01-17 | EXP Services Inc. | London | ON | Type II | industrial |
| 2019-01-18 | Health Sciences North | Sudbury | ON | Type II | medical |
| 2019-01-18 | Health Sciences North | Sudbury | ON | Type II | medical |
| 2019-01-18 | Sudbury Regional Hospital | Sudbury | ON | Type II | academic & research |
| 2019-01-18 | Canadian Blood Services | Brampton | ON | Type II | medical |
| 2019-01-21 | The Ottawa Hospital | Ottawa | ON | Type II | medical |
| 2019-01-21 | The Ottawa Hospital | Ottawa | ON | Type II | medical |
| 2019-01-21 | Inspectrum Testing Inc. | Grande Prairie | AB | Type II | industrial |
| 2019-01-21 | Intrepid NDE Testing Corp. | Grande Prairie | AB | Type II | industrial |
| 2019-01-21 | NOVA Chemicals Corporation | Calgary | AB | Type II | industrial |
| 2019-01-22 | Nortech Advanced N.D.T. Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-01-22 | Gamma Spec NDT Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-01-23 | Nine Energy Canada Inc. | Clairmont | AB | Type II | industrial |
| 2019-01-23 | BJ Services Holdings Canada, ULC | Clairmont | AB | Type II | industrial |
| 2019-01-24 | Core Laboratories Canada Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-01-24 | Canadian Blood Services | Calgary | AB | Type II | medical |
| 2019-01-24 | S.G.H. Inspection Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-01-28 | BAKOSNDT Ltd. | Whitecourt | AB | Type II | industrial |
| 2019-01-28 | MPE Engineering Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-01-29 | Saskatchewan Cancer Agency | Saskatoon | SK | Type II | medical |
| 2019-01-29 | Alco Gas & Oil Production Equipment Ltd. | Edmonton | AB | Type II | industrial |
| 2019-01-29 | Saskatchewan Health Authority | Saskatoon | SK | Type II | medical |
| 2019-01-29 | Saskatchewan Health Authority | Saskatoon | SK | Type II | medical |
| 2019-01-29 | Saskatchewan Health Authority | Saskatoon | SK | Type II | medical |
| 2019-01-30 | Weatherford Canada Ltd. | Red Deer County | AB | Type II | industrial |
| 2019-01-30 | Terracon Geotechnique Ltd. | Nisku | AB | Type II | industrial |
| 2019-01-30 | Rivest Technologies Incorporated | Edmonton | AB | Type II | industrial |
| 2019-01-30 | Acuren Inc. | Edmonton | AB | Type II | industrial |
| 2019-01-30 | Agrium Potash Ltd. | Vanscoy | SK | Type II | industrial |
| 2019-01-30 | Agrium Potash Ltd. | Vanscoy | SK | Type II | industrial |
| 2019-01-31 | IRISNDT Corp. | Edmonton | AB | Type II | industrial |
| 2019-01-31 | Horton CBI, Limited | Sturgeon County | AB | Type II | industrial |
| 2019-01-31 | Clifton Associates Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-01-31 | Canadian Institute for NDE | Hamilton | ON | Type II | industrial |
| 2019-01-31 | Associated Engineering (Sask.) Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-01-31 | Natural Resources Canada | Hamilton | ON | Type II | industrial |
| 2019-02-04 | Royal Military College of Canada | Kingston | ON | Type II | academic & research |
| 2019-02-04 | Invista (Canada) Company | Kingston | ON | Type II | industrial |
| 2019-02-04 | ALS Canada Ltd. | North Vancouver | BC | Type II | academic & research |
| 2019-02-05 | Golder Associates Ltd. | Burnaby | BC | Type II | industrial |
| 2019-02-05 | Queen's University at Kingston | Kingston | ON | Type II | academic & research |
| 2019-02-05 | Queen's University at Kingston | Kingston | ON | Type II | academic & research |
| 2019-02-05 | Queen's University at Kingston | Kingston | ON | Type II | academic & research |
| 2019-02-05 | Queen's University at Kingston | Kingston | ON | Type II | academic & research |
| 2019-02-05 | Klohn Crippen Berger Ltd. | Vancouver | BC | Type II | industrial |
| 2019-02-05 | Strathcona Paper GP Inc. | Napanee | ON | Type II | industrial |
| 2019-02-05 | Kingston General Hospital | Kingston | ON | Type II | academic & research |
| 2019-02-05 | Kingston General Hospital | Kingston | ON | Type II | medical |
| 2019-02-05 | Factory Brewing Ltd. | Vancouver | BC | Type II | industrial |
| 2019-02-06 | Kingston Heart Clinic Nuclear and Vascular Laboratory Inc. | Kingston | ON | Type II | medical |
| 2019-02-06 | Layfield Canada Ltd. | Richmond | BC | Type II | industrial |
| 2019-02-06 | WSP Canada Inc. | Richmond | BC | Type II | industrial |
| 2019-02-06 | Lehigh Cement Company | Picton | ON | Type II | industrial |
| 2019-02-07 | Quinte Healthcare Corporation | Belleville | ON | Type II | medical |
| 2019-02-07 | Quinte Healthcare Corporation | Belleville | ON | Type II | medical |
| 2019-02-07 | Stasuk Testing & Inspection Ltd. | Burnaby | BC | Type II | industrial |
| 2019-02-07 | Sigma Stretch Film of Canada Co. | Belleville | ON | Type II | industrial |
| 2019-02-07 | Geowest Testing Services Ltd. | Burnaby | BC | Type II | industrial |
| 2019-02-07 | Stantec Consulting Ltd. | Burnaby | BC | Type II | industrial |
| 2019-02-07 | Trenergy Inc. | St Catharines | ON | Type II | industrial |
| 2019-02-07 | Mistras Canada, Inc. | Various cities | AB | Type I | industrial |
| 2019-02-08 | Steel Inspection & Testing Ltd. | St Catharines | ON | Type II | industrial |
| 2019-02-08 | Coveright Surfaces Canada Inc. | Cobourg | ON | Type II | industrial |
| 2019-02-11 | Johns Manville Canada Inc. | Innisfail | AB | Type II | industrial |
| 2019-02-11 | Hopewell Designs Inc. | Ottawa | ON | Type II | commercial |
| 2019-02-11 | Step Energy Services Ltd. | Red Deer | AB | Type II | industrial |
| 2019-02-12 | Halton HealthCare Services Corporation | Oakville | ON | Type II | medical |
| 2019-02-12 | Halton HealthCare Services Corporation | Oakville | ON | Type II | medical |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | academic & research |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | academic & research |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | academic & research |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | academic & research |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | commercial |
| 2019-02-12 | University of Calgary | Calgary | AB | Type II | commercial |
| 2019-02-12 | Trillium Health Partners | Toronto | ON | Type II | medical |
| 2019-02-12 | Trillium Health Partners | Toronto | ON | Type II | medical |
| 2019-02-13 | Northwest Nuclear Imaging Limited | Scarborough | ON | Type II | medical |
| 2019-02-13 | 1908273 Ontario Ltd. | Vaughan | ON | Type II | medical |
| 2019-02-13 | Shell Canada Limited | Fort Saskatchewan | AB | Type II | industrial |
| 2019-02-13 | Shell Canada Limited | Fort Saskatchewan | AB | Type II | industrial |
| 2019-02-13 | Shell Canada Limited | Fort Saskatchewan | AB | Type II | industrial |
| 2019-02-14 | Haddad Geotechnical Inc. | Markham | ON | Type II | industrial |
| 2019-02-14 | Cargill Limited | Camrose | AB | Type II | industrial |
| 2019-02-14 | William Osler Health Centre | Toronto | ON | Type II | medical |
| 2019-02-14 | William Osler Health Centre | Toronto | ON | Type II | medical |
| 2019-02-14 | Canadian Dewatering (2006) Ltd. | Edmonton | AB | Type II | industrial |
| 2019-02-14 | SAFFA Engineering Inc. | Markham | ON | Type II | industrial |
| 2019-02-14 | Unity Health Toronto | Toronto | ON | Type II | medical |
| 2019-02-14 | Unity Health Toronto | Toronto | ON | Type II | medical |
| 2019-02-15 | University of Lethbridge | Lethbridge | AB | Type II | academic & research |
| 2019-02-15 | University of Lethbridge | Lethbridge | AB | Type II | academic & research |
| 2019-02-15 | University of Lethbridge | Lethbridge | AB | Type II | academic & research |
| 2019-02-15 | University of Lethbridge | Lethbridge | AB | Type II | academic & research |
| 2019-02-19 | Northern Alberta Institute of Technology | Edmonton | AB | Type II | industrial |
| 2019-02-19 | Terrapex Environment Ltd. | Brossard | QC | Type II | industrial |
| 2019-02-19 | Wright Quality Services Inc. | Beaumont | AB | Type II | industrial |
| 2019-02-20 | Cégep de Sainte-Foy | Québec | QC | Type II | medical |
| 2019-02-20 | Southern Alberta Institute of Technology | Calgary | AB | Type II | industrial |
| 2019-02-20 | C.B. Non-Destructive Testing Ltd | Oakville | ON | Type II | industrial |
| 2019-02-20 | Mistras Canada, Inc. | Red Deer | AB | Type II | industrial |
| 2019-02-20 | Geolog Solutions Inc. | Red Deer County | AB | Type II | industrial |
| 2019-02-20 | Groupe Conseil SCT inc. | Brossard | QC | Type II | industrial |
| 2019-02-20 | Spectrum Wireline Services Ltd. | Red Deer County | AB | Type II | industrial |
| 2019-02-20 | Kingston General Hospital | Kingston | ON | Type II | medical |
| 2019-02-21 | Mistras Canada, Inc. | Various cities | QC, ON | Type I | industrial |
| 2019-02-21 | Big Guns Energy Services Inc. | Red Deer | AB | Type II | industrial |
| 2019-02-21 | Echo NDE Inc. | Red Deer | AB | Type II | industrial |
| 2019-02-21 | Groupe ABS Inc. | Blainville | QC | Type II | industrial |
| 2019-02-22 | NDT Group Inc. | Brantford | ON | Type II | industrial |
| 2019-02-25 | SNC-Lavalin Inc. | Saskatoon | SK | Type II | industrial |
| 2019-02-25 | SNC-Lavalin Inc. | Saskatoon | SK | Type II | academic & research |
| 2019-02-25 | Shaw Pipeline Services Ltd. | Sherwood Park | AB | Type II | industrial |
| 2019-02-26 | Golder Associates Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-02-26 | Golder Associates Ltd. | Saskatoon | SK | Type II | academic & research |
| 2019-02-26 | Potash Corporation of Saskatchewan Inc. | Saskatoon | SK | Type II | industrial |
| 2019-02-26 | Metalogic Inspection Services Inc. | Edmonton | AB | Type II | industrial |
| 2019-02-26 | Tier 1 Energy Solutions, Inc. | Leduc | AB | Type II | industrial |
| 2019-02-27 | ABC Canada Technology Group Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-02-27 | alphaNUCLEAR | Saskatoon | SK | Type II | commercial |
| 2019-02-27 | Canadian Inspection Ltd. | Edmonton | AB | Type II | industrial |
| 2019-02-27 | Agriculture and Agri-Food Canada | Saskatoon | SK | Type II | academic & research |
| 2019-02-28 | P. Machibroda Engineering Ltd. | Saskatoon | SK | Type II | industrial |
| 2019-02-28 | The Ottawa Hospital | Ottawa | ON | Type II | medical |
| 2019-02-28 | The Ottawa Hospital | Ottawa | ON | Type II | medical |
| 2019-02-28 | Sylvia Fedoruk Canadian Centre for Nuclear Innovation Inc. | Saskatoon | SK | Type II | academic & research |
| 2019-02-28 | React Radiography Ltd. | Edmonton | AB | Type II | industrial |
| 2019-02-28 | Canadian Cutting & Coring (Toronto) Ltd | Brampton | ON | Type II | industrial |
| 2019-02-28 | Buffalo Inspection Services (2005) Inc. | Edmonton | AB | Type II | industrial |
| 2019-03-01 | The Ottawa Hospital | Ottawa | ON | Type I | medical |
| 2019-03-05 | 879142 Alberta Ltd. | Calgary | AB | Type II | commercial |
| 2019-03-05 | 879142 Alberta Ltd. | Calgary | AB | Type II | commercial |
| 2019-03-06 | Rainbow Engineering Inc. | Calgary | AB | Type II | industrial |
| 2019-03-06 | Scarborough and Rouge Hospital | Scarborough | ON | Type II | medical |
| 2019-03-06 | Scarborough and Rouge Hospital | Scarborough | ON | Type II | medical |
| 2019-03-07 | BWXT Canada LTD. | Cambridge | ON | Type II | industrial |
| 2019-03-07 | Montreal Neurological Institute | Montreal | QC | Type I | commercial |
| 2019-03-07 | Orbit Engineering Limited | Brampton | ON | Type II | industrial |
| 2019-03-08 | Advance Cardiology Consultants and Diagnostics Inc. | Calgary | AB | Type II | medical |
| 2019-03-08 | Lone Pine Geotechnical Ltd. | Calgary | AB | Type II | industrial |
| 2019-03-11 | The Hospital for Sick Children | Toronto | ON | Type II | academic & research |
| 2019-03-11 | The Hospital for Sick Children | Toronto | ON | Type II | academic & research |
| 2019-03-11 | The Hospital for Sick Children | Toronto | ON | Type II | academic & research |
| 2019-03-11 | The Hospital for Sick Children | Toronto | ON | Type II | academic & research |
| 2019-03-11 | Hunt Inspection Ltd. | Stettler | AB | Type II | industrial |
| 2019-03-12 | Labcan (1989) Ltée | Trois-Rivières | QC | Type II | industrial |
| 2019-03-12 | Tyne Engineering Inc. | Burlington | ON | Type II | academic & research |
| 2019-03-12 | Industrial Radiography Supplies & Services Inc. | Edmonton | AB | Type II | commercial |
| 2019-03-12 | Industrial Radiography Supplies & Services Inc. | Edmonton | AB | Type II | commercial |
| 2019-03-12 | Industrial Radiography Supplies & Services Inc. | Edmonton | AB | Type II | commercial |
| 2019-03-12 | Hoskin Scientific Limited | Burlington | ON | Type II | commercial |
| 2019-03-12 | Hoskin Scientific Limited | Burlington | ON | Type II | commercial |
| 2019-03-12 | G Tech Geotechnical Inc. | Magrath | AB | Type II | industrial |
| 2019-03-13 | Collège d’enseignement général et professsionnel de Trois-Rivières | Trois-Rivières | QC | Type II | industrial |
| 2019-03-13 | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | commercial |
| 2019-03-13 | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | commercial |
| 2019-03-13 | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | commercial |
| 2019-03-13 | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | commercial |
| 2019-03-13 | Chinook Regional Hospital | Lethbridge | AB | Type II | medical |
| 2019-03-13 | Chinook Regional Hospital | Lethbridge | AB | Type II | medical |
| 2019-03-13 | Alpha Adroit Engineering Ltd. | Edmonton | AB | Type II | industrial |
| 2019-03-13 | Centre intégré universitaire de santé et de services sociaux du Nord-de-l'Île-de-Montréal | Montréal | QC | Type II | medical |
| 2019-03-13 | Centre intégré universitaire de santé et de services sociaux du Nord-de-l'Île-de-Montréal | Montréal | QC | Type II | medical |
| 2019-03-14 | University of Waterloo | Waterloo | ON | Type II | academic & research |
| 2019-03-14 | University of Waterloo | Waterloo | ON | Type II | academic & research |
| 2019-03-14 | University of Waterloo | Waterloo | ON | Type II | academic & research |
| 2019-03-14 | University of Waterloo | Waterloo | ON | Type II | academic & research |
| 2019-03-14 | University of Waterloo | Waterloo | ON | Type II | academic & research |
| 2019-03-14 | Canadoil Forge Ltd. | Bécancour | QC | Type II | industrial |
| 2019-03-14 | Wood Canada Limited | Edmonton | AB | Type II | industrial |
| 2019-03-14 | Maxxam Analytics International Corporation | Mississauga | ON | Type II | academic & research |
| 2019-03-15 | Kubota Materials Canada Corporation | Orillia | ON | Type II | industrial |
| 2019-03-15 | Kubota Materials Canada Corporation | Orillia | ON | Type II | industrial |
| 2019-03-15 | Les Inspections Thermetco Inc. | Montréal | QC | Type II | industrial |
| 2019-03-15 | DGI Geoscience Inc. | Barrie | ON | Type II | industrial |
| 2019-03-15 | Central Alberta Medical Imaging Services Limited | Red Deer | AB | Type II | medical |
| 2019-03-15 | GeoTerre Limited | Brampton | ON | Type II | industrial |
| 2019-03-18 | NCL Envirotek Inc. | Montréal | QC | Type II | industrial |
| 2019-03-19 | University of Toronto | Toronto | ON | Type II | commercial |
| 2019-03-19 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Sainte-Marie | QC | Type II | medical |
| 2019-03-19 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Sainte-Marie | QC | Type II | medical |
| 2019-03-20 | Perfection Inspection Limited | Cambridge | ON | Type II | industrial |
| 2019-03-20 | Perfection Inspection Limited | Cambridge | ON | Type II | industrial |
| 2019-03-20 | Unique Detection Services Limited | Cambridge | ON | Type II | industrial |
| 2019-03-20 | Shell Canada Limited | Caroline | AB | Type II | industrial |
| 2019-03-20 | EnergySolutions Canada | Brampton | ON | Type II | commercial |
| 2019-03-21 | EnergySolutions Canada | Concord | ON | Type II | commercial |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | academic & research |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | academic & research |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | academic & research |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | medical |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | medical |
| 2019-03-21 | Mount Sinai Hospital | Toronto | ON | Type II | medical |
| 2019-03-21 | Cégep de Sainte-Foy | Québec | QC | Type II | medical |
| 2019-03-21 | Ontario Power Generation Inc. | Pickering | ON | Type II | industrial |
| 2019-03-21 | 860851 Alberta Ltd. | Drayton Valley | AB | Type II | industrial |
| 2019-03-21 | McElhanney Consulting Services Ltd. | Courtenay | BC | Type II | industrial |
| 2019-03-21 | McElhanney Consulting Services Ltd. | Campbell River | BC | Type II | industrial |
| 2019-03-22 | Lewkowich Engineering Associates Ltd. | Courtenay | BC | Type II | industrial |
| 2019-03-22 | McElhanney Consulting Services Ltd. | Courtenay | BC | Type II | industrial |
| 2019-03-22 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Lévis | QC | Type II | medical |
| 2019-03-25 | University of Regina | Regina | SK | Type II | academic & research |
| 2019-03-25 | University of Regina | Regina | SK | Type II | academic & research |
| 2019-03-25 | University of Regina | Regina | SK | Type II | academic & research |
| 2019-03-26 | Horton CBI, Limited | Regina | SK | Type II | industrial |
| 2019-03-26 | Les Laboratoires d'Essais Mequaltech Inc. | Sherbrooke | QC | Type II | industrial |
| 2019-03-26 | TISI Canada Inc. | Regina | SK | Type II | industrial |
| 2019-03-26 | TISI Canada Inc. | Regina | SK | Type II | industrial |
| 2019-03-26 | The Graff Company Ltd. | Mississauga | ON | Type II | industrial |
| 2019-03-26 | Ezeflow Inc. | Granby | QC | Type II | industrial |
| 2019-03-26 | Ezeflow Inc. | Granby | QC | Type II | industrial |
| 2019-03-27 | Proco Technical Services Ltd. | Yorkton | SK | Type II | industrial |
| 2019-03-27 | Granulab Inc. | Sherbrooke | QC | Type II | industrial |
| 2019-03-27 | Englobe Corp. | Laval | QC | Type II | industrial |
| 2019-03-27 | Fedorowich Construction Ltd. | Yorkton | SK | Type II | industrial |
| 2019-03-27 | Centre intégré de santé et de services sociaux de la Montérégie-Centre | Greenfield Park | QC | Type II | medical |
| 2019-03-27 | Centre intégré de santé et de services sociaux de la Montérégie-Centre | Greenfield Park | QC | Type II | medical |
| 2019-03-28 | McGill University Health Centre | Montréal | QC | Type II | medical |
| 2019-03-28 | McGill University Health Centre | Montréal | QC | Type II | medical |
| 2019-03-28 | Coco Paving Inc. | Regina | SK | Type II | industrial |
| 2019-03-28 | Tetra Tech Canada Inc. | Regina | SK | Type II | industrial |
| 2019-04-01 | Cornwall Community Hospital | Cornwall | ON | Type II | medical |
| 2019-04-01 | Cornwall Community Hospital | Cornwall | ON | Type II | medical |
| 2019-04-02 | TotalCardiology Services Inc. | Calgary | AB | Type II | medical |
| 2019-04-02 | Uni-Tech Inspection Services Ltd. | South Glengarry | ON | Type II | industrial |
| 2019-04-02 | Uni-Tech Inspection Services Ltd. | South Glengarry | ON | Type II | industrial |
| 2019-04-02 | Teknoscan Systems Inc. | Vaughan | ON | Type II | commercial |
| 2019-04-02 | Valmet Ltd. | Vaughan | ON | Type II | commercial |
| 2019-04-02 | National Research Council of Canada | Ottawa | ON | Type II | academic & research |
| 2019-04-02 | National Research Council of Canada | Ottawa | ON | Type II | academic & research |
| 2019-04-02 | National Research Council of Canada | Ottawa | ON | Type II | academic & research |
| 2019-04-02 | National Research Council of Canada | Ottawa | ON | Type II | academic & research |
| 2019-04-03 | Procter & Gamble Inc | Brockville | ON | Type II | industrial |
| 2019-04-03 | Trillium Health Care Products Inc. | Brockville | ON | Type II | industrial |
| 2019-04-04 | Croft Engineering Ltd. | Cochrane | AB | Type II | industrial |
| 2019-04-08 | CancerCare Manitoba | Winnipeg | MB | Type II | medical |
| 2019-04-08 | CancerCare Manitoba | Winnipeg | MB | Type II | commercial |
| 2019-04-08 | CancerCare Manitoba | Winnipeg | MB | Type II | commercial |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | academic & research |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | academic & research |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-09 | Winnipeg Regional Health Authority | Winnipeg | MB | Type II | medical |
| 2019-04-10 | Canada Border Services Agency | Emerson | MB | Type II | industrial |
| 2019-04-10 | Canada Border Services Agency | Emerson | MB | Type II | REMOVE |
| 2019-04-10 | Bunge Canada Holdings I ULC | Altona | MB | Type II | industrial |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-11 | University of Manitoba | Winnipeg | MB | Type II | academic & research |
| 2019-04-12 | M. Block & Associates Limited | Winnipeg | MB | Type II | industrial |
| 2019-04-15 | McIntosh Perry Limited | Woodbridge | ON | Type II | industrial |
| 2019-04-15 | McIntosh Perry Limited | Woodbridge | ON | Type II | industrial |
| 2019-04-16 | Omya (Canada) Inc. | Perth | ON | Type II | industrial |
| 2019-04-16 | KMH Cardiology Centres Incorporated | Hamilton | ON | Type II | medical |
| 2019-04-16 | Aecom Canada Ltd. | Etobicoke | ON | Type II | industrial |
| 2019-04-16 | 3M Canada Company | Perth | ON | Type II | industrial |
| 2019-04-17 | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | ON | Type II | commercial |
| 2019-04-18 | Golder Associates Ltd. | Ottawa | ON | Type II | industrial |
| 2019-04-18 | University of Ottawa Health Institute | Ottawa | ON | Type I | commercial |
| 2019-04-18 | Kilborn Nuclear Imaging Inc. | Ottawa | ON | Type II | medical |
| 2019-04-18 | Stantec Consulting Ltd. | Laval | QC | Type II | industrial |
| 2019-04-18 | Construction DJL Inc./ | Laval | QC | Type II | industrial |
| 2019-04-23 | Lafarge Canada Inc. | Exshaw | AB | Type II | industrial |
| 2019-04-23 | Tomlinson Enterprises Ltd. | Sarnia | ON | Type II | industrial |
| 2019-04-23 | Tomlinson Enterprises Ltd. | Sarnia | ON | Type II | industrial |
| 2019-04-23 | Thermo Gamma-Metrics LLC | Ottawa | ON | Type II | commercial |
| 2019-04-23 | Canadian Tower Scanning Inc. | Sarnia | ON | Type II | industrial |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | University of Windsor | Fort McMurray | AB | Type II | academic & research |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | University of Windsor | Windsor | ON | Type II | academic & research |
| 2019-04-24 | Interface Testing Services Inc. | Sarnia | ON | Type II | industrial |
| 2019-04-24 | DB Ground Testing Ltd. | Agassiz | BC | Type II | industrial |
| 2019-04-24 | Canadian Construction Materials Engineering & Testing Inc. | Calgary | AB | Type II | industrial |
| 2019-04-24 | GeoWest Engineering Ltd. | Abbotsford | BC | Type II | industrial |
| 2019-04-24 | Kinectrics Inc. | Tiverton | ON | Type II | commercial |
| 2019-04-25 | Vancouver Coastal Health Authority | Abbotsford | BC | Type II | medical |
| 2019-04-25 | Vancouver Coastal Health Authority | Abbotsford | BC | Type II | medical |
| 2019-04-25 | Xenon Pharmaceuticals Inc. | Burnaby | BC | Type II | academic & research |
| 2019-04-25 | Nuclear Services Canada Inc. | Merlin | ON | Type II | commercial |
| 2019-04-25 | Nuclear Services Canada Inc. | Merlin | ON | Type II | commercial |
| 2019-04-25 | Kinectrics Inc. | Teeswater | ON | Type II | commercial |
| 2019-04-26 | Highway Innovation Inc. | Calgary | AB | Type II | industrial |
| 2019-04-26 | Kinectrics Inc. | Toronto | ON | Type II | commercial |
| 2019-05-03 | Winnipeg Regional Health Authority | Winnipeg | ON | Type I | commercial |
| 2019-05-03 | Big Rock Brewery Ltd. | Calgary | AB | Type II | industrial |
| 2019-05-07 | Atlas Testing Labs & Services (Nova Scotia) Ltd. | Salt Springs | NS | Type II | industrial |
| 2019-05-07 | Goodyear Canada Inc. | Salaberry-de-Valleyfield | QC | Type II | industrial |
| 2019-05-07 | Centre intégré de santé et de services sociaux de la Montérégie-Centre | St-Jean-sur-Richelieu | QC | Type II | medical |
| 2019-05-07 | Centre intégré de santé et de services sociaux de la Montérégie-Centre | St-Jean-sur-Richelieu | QC | Type II | medical |
| 2019-05-07 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Longueuil | QC | Type II | medical |
| 2019-05-07 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Longueuil | QC | Type II | medical |
| 2019-05-07 | MyHealth Partners Inc. | Toronto | ON | Type II | medical |
| 2019-05-07 | MyHealth Partners Inc. | Scarborough | ON | Type II | medical |
| 2019-05-08 | P. Baillargeon Ltée | St-Jean-Richelieu | QC | Type II | industrial |
| 2019-05-08 | ALI Excavation Inc. | Salaberry-de-Valleyfield | QC | Type II | industrial |
| 2019-05-08 | Citoxlab Amérique du Nord Inc. | Laval | QC | Type II | academic & research |
| 2019-05-08 | Groupe Conseil SCT inc. | St-Jean-sur-Richelieu | QC | Type II | industrial |
| 2019-05-08 | Centre intégré universitaire de santé et de services sociaux de l'Ouest-de-l'Ìle-de-Montréal | Pointe-Claire | QC | Type II | medical |
| 2019-05-08 | Centre intégré universitaire de santé et de services sociaux de l'Ouest-de-l'Ìle-de-Montréal | Pointe-Claire | QC | Type II | medical |
| 2019-05-08 | MyHealth Partners Inc. | Stouffville | ON | Type II | medical |
| 2019-05-08 | RTD Quality Services Inc. | Saint John | NB | Type II | Industrial |
| 2019-05-09 | ABB Inc. | St-Laurent | QC | Type II | commercial |
| 2019-05-09 | Formica Canada Inc. | St-Jean-sur-Richelieu | QC | Type II | industrial |
| 2019-05-09 | Centre Intégré de Santé et de Services Sociaux de Laval | Laval | QC | Type II | medical |
| 2019-05-09 | Centre Intégré de Santé et de Services Sociaux de Laval | Laval | QC | Type II | medical |
| 2019-05-09 | Baker Hughes | Mount Pearl | NL | Type II | industrial |
| 2019-05-10 | MyHealth Partners Inc. | Various cities | ON | Type I | medical |
| 2019-05-10 | Curtis Geo Solutions Inc. | Calgary | AB | Type II | industrial |
| 2019-05-13 | Malroz Engineering Inc. | Kingston | ON | Type II | industrial |
| 2019-05-15 | Bubble Technology Industries Inc. | Chalk River | ON | Type II | industrial |
| 2019-05-17 | Bubble Technology Industries Inc. | Chalk River | ON | Type II | industrial |
| 2019-05-22 | Civil ArSa Engineering Inc. | Queensville | ON | Type II | industrial |
| 2019-05-22 | Trek Geotechnical Inc. | Winnipeg | MB | Type II | industrial |
| 2019-05-22 | Gerdau Ameristeel Corporation | Selkirk | MB | Type II | industrial |
| 2019-05-22 | Noveen Engineering Inc. | Georgina | ON | Type II | industrial |
| 2019-05-23 | Tantalum Mining Corporation of Canada Ltd. | Lac du Bonnet | MB | Type II | industrial |
| 2019-05-23 | Englobe Corp. | Calgary | AB | Type II | industrial |
| 2019-05-27 | Englobe Corp. | Calgary | AB | Type II | industrial |
| 2019-05-28 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-05-28 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-05-28 | Alberta Health Services | Calgary | AB | Type II | academic & research |
| 2019-05-28 | Alberta Health Services | Calgary | AB | Type II | commercial |
| 2019-05-28 | Lewkowich Engineering Associates Ltd. | Nanaimo | BC | Type II | industrial |
| 2019-05-28 | Wood Canada Limited | Red Deer | AB | Type II | industrial |
| 2019-05-28 | Union Street Geotechnical Ltd. | Red Deer | AB | Type II | industrial |
| 2019-05-28 | Tetra Tech Canada Inc. | Nanaimo | BC | Type II | industrial |
| 2019-05-28 | Scarborough Health Network | Scarborough | ON | Type II | medical |
| 2019-05-28 | Scarborough and Rouge Hospital | Scarborough | ON | Type II | medical |
| 2019-05-28 | Formosa Springs Brewery Inc. | Formosa | ON | Type II | industrial |
| 2019-05-29 | Toronto East Health Network | Toronto | ON | Type II | medical |
| 2019-05-29 | Toronto East Health Network | Toronto | ON | Type II | medical |
| 2019-05-29 | Canadian Construction Materials Engineering & Testing Inc. | Courtenay | BC | Type II | industrial |
| 2019-05-30 | Sarafinchin Associates Ltd. | Toronto | ON | Type II | industrial |
| 2019-05-30 | Nine Energy Canada Inc. | Red Deer | AB | Type II | industrial |
| 2019-05-30 | SNC-Lavalin GEM Québec Inc. | Laval | QC | Type II | industrial |
| 2019-05-30 | SNC-Lavalin GEM Québec Inc. | Laval | QC | Type II | industrial |
| 2019-05-30 | EXP Services Inc. | Laval | QC | Type II | industrial |
| 2019-05-31 | Kodiak Nondestructive Testing Services Ltd. | Nanaimo | BC | Type II | industrial |
| 2019-06-04 | Goldcorp Canada Ltd. | Balmertown | ON | Type II | industrial |
| 2019-06-04 | Goldcorp Canada Ltd. | Balmertown | ON | Type II | industrial |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | academic & research |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-04 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-05 | Pioneer Construction Inc. | Kenora | ON | Type II | industrial |
| 2019-06-05 | Grey Nuns Community Health Centre | Edmonton | AB | Type II | medical |
| 2019-06-05 | Covenant Health | Edmonton | AB | Type II | medical |
| 2019-06-05 | Medical Imaging Consultants | Edmonton | AB | Type II | medical |
| 2019-06-05 | Miller Northwest Limited | Dryden | ON | Type II | industrial |
| 2019-06-05 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-05 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-05 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-05 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | medical |
| 2019-06-05 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | medical |
| 2019-06-05 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | medical |
| 2019-06-05 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | medical |
| 2019-06-06 | Cascades Canada ULC | Trenton | ON | Type II | industrial |
| 2019-06-06 | Keewatin-Aski Ltd. | Sioux Lookout | ON | Type II | industrial |
| 2019-06-06 | Domtar Inc. | Dryden | ON | Type II | industrial |
| 2019-06-06 | Fidelity Engineering & Construction Inc. | Colborne | ON | Type II | industrial |
| 2019-06-06 | Natural Resources Canada | Devon | AB | Type II | industrial |
| 2019-06-07 | DST Consulting Engineers Inc. | Kenora | ON | Type II | industrial |
| 2019-06-07 | Trenergy Inc. | St Catharines | ON | Type II | industrial |
| 2019-06-07 | Venture Steel | Toronto | ON | Type II | industrial |
| 2019-06-07 | Venture Steel | Rexdale | ON | Type II | industrial |
| 2019-06-12 | McMaster University | Hamilton | ON | Type II | commercial |
| 2019-06-13 | Regional Health Authority B | Moncton | NB | Type II | medical |
| 2019-06-14 | McMaster University | Hamilton | ON | Type II | academic & research |
| 2019-06-14 | Hamilton Health Sciences Corporation | Hamilton | ON | Type II | medical |
| 2019-06-14 | Centre for Probe Developmnet and Commercialization | Hamilton | ON | Type II | commercial |
| 2019-06-17 | Stantec Consulting Ltd. | Moncton | NB | Type II | industrial |
| 2019-06-17 | WSP Canada Inc. | Val d’Or | QC | Type II | industrial |
| 2019-06-17 | Integra Gold (Québec) Inc. | Val d'Or | QC | Type II | industrial |
| 2019-06-18 | Regional Health Authority A | Bathurst | NB | Type II | medical |
| 2019-06-18 | Clifton Associates Ltd. | Calgary | AB | Type II | industrial |
| 2019-06-18 | St-Isidore Asphalte Ltée | St-Isidore | NB | Type II | industrial |
| 2019-06-18 | Vale Newfoundland & Labrador Limited | Long Harbour | NL | Type II | industrial |
| 2019-06-18 | Aecom Canada Ltd. | Etobicoke | ON | Type II | industrial |
| 2019-06-18 | Regional Health Authority B | Moncton | NB | Type II | medical |
| 2019-06-18 | Breakwater Resources Ltd. | Lebel-sur-Quévillon | QC | Type II | industrial |
| 2019-06-18 | Centre intégré de santé et de services sociaux de l'Abitibi-Témiscamingue | Val d'Or | QC | Type II | medical |
| 2019-06-18 | Labatt Brewing Company Ltd. | St. John's | NL | Type II | industrial |
| 2019-06-18 | Riverview Animal Hospital | Riverview | NB | Type II | medical |
| 2019-06-19 | Ontario Ministry of Labour | Toronto | ON | Type II | academic & research |
| 2019-06-19 | Regional Health Authority A | Bathurst | NB | Type II | medical |
| 2019-06-19 | Central Regional Integrated Health Authority | Gander | NL | Type II | medical |
| 2019-06-19 | IAMGOLD Corporation | Preissac | QC | Type II | industrial |
| 2019-06-19 | DMG Consulting Limited | Gander | NL | Type II | industrial |
| 2019-06-19 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Sorel-Tracy | QC | Type II | medical |
| 2019-06-19 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Sorel-Tracy | QC | Type II | medical |
| 2019-06-19 | North American Lithium Inc. | La Corne | QC | Type II | industrial |
| 2019-06-20 | Canadian Malartic GP | Malartic | QC | Type II | industrial |
| 2019-06-20 | Resolute FP Canada Inc. | Amos | QC | Type II | industrial |
| 2019-06-20 | Halliburton Canada | Mount Pearl | NL | Type II | industrial |
| 2019-06-20 | AV Group NB Inc. | Atholville | NB | Type II | industrial |
| 2019-06-20 | Gemtec Consulting Engineers and Scientists Limited | Bathurst | NB | Type II | industrial |
| 2019-06-20 | Schlumberger Canada Limited | Mount Pearl | NL | Type II | industrial |
| 2019-06-20 | Schlumberger Canada Limited | Mount Pearl | NL | Type II | industrial |
| 2019-06-21 | RTD Quality Services Inc. | Bathurst | NB | Type II | industrial |
| 2019-06-21 | OneSubsea Canada ULC | Mount Pearl | NL | Type II | commercial |
| 2019-06-25 | Berthold Technologies U.S.A., LLC | Montréal | QC | Type II | commercial |
| 2019-06-25 | Tafisa Canada Inc. | Lac-Mégantic | QC | Type II | industrial |
| 2019-06-26 | City of Calgary | Calgary | AB | Type II | industrial |
| 2019-06-26 | Spectrum NDT Ltd. | Calgary | AB | Type II | industrial |
| 2019-06-26 | CIUSSS de l'Estrie - CHUS | Fleurimont | QC | Type II | medical |
| 2019-06-26 | CIUSSS de l'Estrie - CHUS | Fleurimont | QC | Type II | medical |
| 2019-06-26 | CIUSSS de l'Estrie - CHUS | Fleurimont | QC | Type II | medical |
| 2019-06-26 | Emballages Novus Inc. | Richmond | QC | Type II | industrial |
| 2019-06-27 | SNC-Lavalin GEM Québec Inc. | Montréal | QC | Type II | industrial |
| 2019-06-27 | Construction DJL Inc. | Bromont | QC | Type II | industrial |
| 2019-06-27 | Les Entreprises Michaudville Inc | Mont-Saint-Hilaire | QC | Type II | industrial |
| 2019-06-30 | OneSubsea Canada ULC | Mount Pearl | NL | Type II | commercial |
| 2019-07-03 | Boss Wireline Services Ltd. | Brooks | AB | Type II | industrial |
| 2019-07-03 | GEM Testing Ltd. | Dunmore | AB | Type II | industrial |
| 2019-07-04 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | commercial |
| 2019-07-04 | Reliance OFS Canada Ltd. | Redcliff | AB | Type II | industrial |
| 2019-07-04 | Vision Integrity Engineering Ltd. | Medicine Hat | AB | Type II | industrial |
| 2019-07-04 | Vision Integrity Engineering Ltd. | Medicine Hat | AB | Type II | industrial |
| 2019-07-05 | Medicine Hat Regional Hospital | Medicine Hat | AB | Type II | medical |
| 2019-07-05 | Medicine Hat Regional Hospital | Medicine Hat | AB | Type II | medical |
| 2019-07-05 | Centre intégré de santé et de services sociaux de l'Outaouais | Gatineau | QC | Type II | commercial |
| 2019-07-08 | Nortech Advanced N.D.T. Ltd. | Fort St. John | BC | Type II | industrial |
| 2019-07-08 | Stantec Consulting Ltd. | Saint John | NB | Type II | industrial |
| 2019-07-08 | Acuren Inc. | Castlegar | BC | Type II | industrial |
| 2019-07-08 | Gemtec Consulting Engineers and Scientists Limited | Saint John | NB | Type II | industrial |
| 2019-07-09 | Canadian Light Source | Saskatoon | SK | Type II | academic & research |
| 2019-07-09 | Irving Pulp and Paper Limited | Saint John | NB | Type II | industrial |
| 2019-07-09 | Sarafinchin Associates Ltd. | Toronto | ON | Type II | industrial |
| 2019-07-09 | Kootenay Boundary Regional Hospital | Trail | BC | Type II | medical |
| 2019-07-09 | Kootenay Boundary Regional Hospital | Trail | BC | Type II | medical |
| 2019-07-09 | Zellstoff Celgar Limited | Castlegar | BC | Type II | industrial |
| 2019-07-09 | Atomic Inspection Services Ltd. | Fort St. John | BC | Type II | industrial |
| 2019-07-09 | Custom Fabricators & Machinists Limited | Saint John | NB | Type II | industrial |
| 2019-07-09 | Deka Inspection Services Ltd. | Charlie Lake | BC | Type II | industrial |
| 2019-07-09 | Black Swan Energy Ltd. | Wonowon | BC | Type II | industrial |
| 2019-07-10 | Lake Utopia Paper, Division of J.D. Irving Ltd. | Utopia | NB | Type II | industrial |
| 2019-07-10 | University of British Columbia | Balfour | BC | Type II | academic & research |
| 2019-07-10 | Glade Materials Testing Ltd. | Castlegar | BC | Type II | industrial |
| 2019-07-10 | Allnorth Consultants Limited | Fort NElson | BC | Type II | industrial |
| 2019-07-10 | Owl Inspection Services Ltd. | Fort St. John | BC | Type II | industrial |
| 2019-07-10 | Brunswick Engineering & Consulting Inc. | Saint John | NB | Type II | industrial |
| 2019-07-10 | Gemtec Consulting Engineers and Scientists Limited | Saint John | NB | Type II | industrial |
| 2019-07-10 | Alexander Arthur Werner Winkelmann o/a Central Earth Engineering | Barrie | ON | Type II | industrial |
| 2019-07-10 | Alexander Arthur Werner Winkelmann o/a Central Earth Engineering | Barrie | ON | Type II | industrial |
| 2019-07-11 | RTD Quality Services Inc. | Saint John | NB | Type II | industrial |
| 2019-07-11 | Northern Health Authority | Fort St. John | BC | Type II | medical |
| 2019-07-11 | Northern Health Authority | Fort St. John | BC | Type II | medical |
| 2019-07-11 | Kootenay Materials Testing Ltd. | Castlegar | BC | Type II | industrial |
| 2019-07-11 | Acuren Inc. | Saint John | NB | Type II | industrial |
| 2019-07-11 | Acuren Inc. | Saint John | NB | Type II | industrial |
| 2019-07-11 | 1068648 B.C. Ltd. | Fort St. John | BC | Type II | industrial |
| 2019-07-12 | Conquest Engineering Ltd. | Saint John | NB | Type II | industrial |
| 2019-07-15 | Ministère des Transports | Mont-Joli | QC | Type II | industrial |
| 2019-07-15 | Headwaters Health Care Centre | Orangeville | ON | Type II | medical |
| 2019-07-15 | Alliston Diagnostic Centre Inc. | Alliston | ON | Type II | medical |
| 2019-07-15 | Construction DJL Inc. | Mont-Joli | QC | Type II | industrial |
| 2019-07-16 | Rayonier A.M. Canada Enterprises Inc. | Matane | QC | Type II | industrial |
| 2019-07-16 | Les Pavages des Monts Inc. | Matane | QC | Type II | industrial |
| 2019-07-16 | Cardiovascular Care Centre Inc. | Mississauga | ON | Type II | medical |
| 2019-07-16 | Les Entreprises Mont Sterling Inc. | Sainte-Anne-des-Monts | QC | Type II | industrial |
| 2019-07-16 | McIntosh Perry Limited | Woodbridge | ON | Type II | industrial |
| 2019-07-17 | 2352767 Ontario Inc. | North York | ON | Type II | medical |
| 2019-07-17 | Construction DJL Inc. | Gaspé | QC | Type II | industrial |
| 2019-07-17 | Zellstoff Celgar Limited | Chandler | QC | Type II | medical |
| 2019-07-17 | Centre intégré de santé et de services sociaux de la Gaspésie | Chandler | QC | Type II | medical |
| 2019-07-17 | Centre intégré de santé et de services sociaux de la Gaspésie | Gaspé | QC | Type II | medical |
| 2019-07-18 | Southlake Regional Health Centre | Newmarket | ON | Type II | medical |
| 2019-07-18 | Southlake Regional Health Centre | Newmarket | ON | Type II | medical |
| 2019-07-18 | Southlake Regional Health Centre | Newmarket | ON | Type II | medical |
| 2019-07-18 | North York General Hospital | North York | ON | Type II | medical |
| 2019-07-18 | North York General Hospital | North York | ON | Type II | medical |
| 2019-07-18 | Construction DJL Inc. | Grande-Cascapédia | QC | Type II | industrial |
| 2019-07-18 | Centre intégré de santé et de services sociaux de la Gaspésie | Maria | QC | Type II | medical |
| 2019-07-19 | GHD Consultants Ltd. | Mississauga | ON | Type II | industrial |
| 2019-07-22 | DST Consulting Engineers Inc. | Sudbury | ON | Type II | industrial |
| 2019-07-22 | J.& P. Leveque Bros. Haulage Ltd. | French River | ON | Type II | industrial |
| 2019-07-23 | Teranorth Construction & Engineering Limited | Sudbury | ON | Type II | industrial |
| 2019-07-23 | TISI Canada Inc. | Sudbury | ON | Type II | industrial |
| 2019-07-23 | Aecom Canada Ltd. | Sudbury | ON | Type II | industrial |
| 2019-07-23 | Cave Inspection Ltd. | Lloydminster | AB | Type II | industrial |
| 2019-07-23 | Denis Gratton Construction | Sudbury | ON | Type II | industrial |
| 2019-07-24 | Tulloch Contract Administration Inc. | Wahnapitae | ON | Type II | industrial |
| 2019-07-24 | Tulloch Contract Administration Inc. | Wahnapitae | ON | Type II | industrial |
| 2019-07-24 | Teranorth Construction & Engineering Limited | Sudbury | ON | Type II | industrial |
| 2019-07-25 | Englobe Corp. | Edmonton | AB | Type II | industrial |
| 2019-07-25 | Allnorth Consultants Limited | Edmonton | AB | Type II | industrial |
| 2019-07-25 | Allnorth Consultants Limited | Edmonton | AB | Type II | industrial |
| 2019-07-29 | Honeywell Ltd | North Vancouver | BC | Type II | commercial |
| 2019-07-29 | Honeywell Ltd | North Vancouver | BC | Type II | commercial |
| 2019-07-30 | University of British Columbia | Vancouver | BC | Type II | academic & research |
| 2019-07-30 | University of British Columbia | Vancouver | BC | Type II | academic & research |
| 2019-07-30 | University of British Columbia | Vancouver | BC | Type II | academic & research |
| 2019-07-30 | University of British Columbia | Vancouver | BC | Type II | medical |
| 2019-07-30 | Voltage Wireline Inc. | Blackfalds | AB | Type II | industrial |
| 2019-07-30 | M & B Technical Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-07-30 | M & B Technical Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-07-31 | University of Guelph | Guelph | ON | Type II | medical |
| 2019-07-31 | University of Guelph | Guelph | ON | Type II | academic & research |
| 2019-07-31 | University of Guelph | Guelph | ON | Type II | academic & research |
| 2019-07-31 | University of Guelph | Guelph | ON | Type II | commercial |
| 2019-07-31 | Stasuk Testing & Inspection Ltd. | Burnaby | BC | Type II | industrial |
| 2019-07-31 | Almadon Holdings Ltd. | Calgary | AB | Type II | medical |
| 2019-07-31 | BWXT ITG Canada Inc. | Vancouver | BC | Type II | commercial |
| 2019-07-31 | British Columbia Cancer Agency | Vancouver | BC | Type II | academic & research |
| 2019-07-31 | British Columbia Cancer Agency | Vancouver | BC | Type II | academic & research |
| 2019-07-31 | British Columbia Cancer Agency | Vancouver | BC | Type II | medical |
| 2019-07-31 | British Columbia Cancer Agency | Vancouver | BC | Type II | medical |
| 2019-08-01 | IRISNDT Corp. | Red Deer | AB | Type II | industrial |
| 2019-08-01 | McIntosh Lalani Engineering Ltd. | Calgary | AB | Type II | industrial |
| 2019-08-01 | TISI Canada Inc. | Red Deer | AB | Type II | industrial |
| 2019-08-01 | Tetra Tech Canada Inc. | Calgary | AB | Type II | industrial |
| 2019-08-01 | British Columbia Cancer Agency | Vancouver | BC | Type II | medical |
| 2019-08-02 | Aecon Construction and Materials Limited | Caledon | ON | Type II | industrial |
| 2019-08-04 | Coeur Silvertip Holdings Ltd. | Type II | industrial | ||
| 2019-08-04 | Coeur Silvertip Holdings Ltd. | Vancouver | BC | Type II | industrial |
| 2019-08-04 | Coeur Silvertip Holdings Ltd. | Vancouver | BC | Type II | industrial |
| 2019-08-05 | Government of Yukon | Whitehorse | YT | Type II | industrial |
| 2019-08-06 | Dr. Roy Park | Calgary | AB | Type II | medical |
| 2019-08-07 | M & B Technical Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-08-08 | EXP Services Inc. | Calgary | AB | Type II | industrial |
| 2019-08-12 | John D. Paterson & Associates Ltd. | North Bay | ON | Type II | industrial |
| 2019-08-12 | Agriculture and Agri-Food Canada | Fredericton | NB | Type II | academic & research |
| 2019-08-13 | Almor Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-08-13 | Almor Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-08-13 | Almor Testing Services Ltd. | Calgary | AB | Type II | industrial |
| 2019-08-13 | Fabrene Inc. | North Bay | ON | Type II | industrial |
| 2019-08-13 | Miller Group Inc. | North Bay | ON | Type II | industrial |
| 2019-08-13 | Miller Group Inc. | North Bay | ON | Type II | industrial |
| 2019-08-13 | Royal Inland Hospital | Kamloops | BC | Type II | medical |
| 2019-08-13 | Royal Inland Hospital | Kamloops | BC | Type II | medical |
| 2019-08-13 | Dawson Construction Limited | Kamloops | BC | Type II | industrial |
| 2019-08-13 | New Brunswick Power Nuclear Corporation | Fredericton | NB | Type II | academic & research |
| 2019-08-13 | Gemtec Consulting Engineers and Scientists Limited | Fredericton | NB | Type II | industrial |
| 2019-08-13 | Gemtec Consulting Engineers and Scientists Limited | Fredericton | NB | Type II | industrial |
| 2019-08-13 | EXP Services Inc. | Oromocto | NB | Type II | industrial |
| 2019-08-14 | Kingston Health Sciences Centre | Kingston | ON | Type II | medical |
| 2019-08-14 | IRISNDT Corp. | Calgary | AB | Type II | industrial |
| 2019-08-14 | Shaba Testing Services Ltd. | Kirkland Lake | ON | Type II | industrial |
| 2019-08-14 | Shaba Testing Services Ltd. | Kirkland Lake | ON | Type II | industrial |
| 2019-08-14 | Kirkland Lake Gold Inc. | Kirkland Lake | ON | Type II | industrial |
| 2019-08-14 | Allnorth Consultants Limited | Kamloops | BC | Type II | industrial |
| 2019-08-14 | Stantec Consulting Ltd. | Fredericton | NB | Type II | industrial |
| 2019-08-14 | Telford Geotechnical Ltd. | Kamloops | BC | Type II | industrial |
| 2019-08-14 | Canadian Construction Materials Engineering & Testing Inc. | Salmon Arm | BC | Type II | industrial |
| 2019-08-14 | Conquest Engineering Ltd. | Fredericton | NB | Type II | industrial |
| 2019-08-15 | Children's Hospital of Eastern Ontario | Ottawa | ON | Type II | medical |
| 2019-08-15 | Children's Hospital of Eastern Ontario | Ottawa | ON | Type II | academic & research |
| 2019-08-15 | Children's Hospital of Eastern Ontario | Ottawa | ON | Type II | medical |
| 2019-08-15 | Regional Health Authority B | Fredericton | NB | Type II | medical |
| 2019-08-15 | Regional Health Authority B | Fredericton | NB | Type II | medical |
| 2019-08-15 | University of New Brunswick | Fredericton | NB | Type II | academic & research |
| 2019-08-15 | University of New Brunswick | Fredericton | NB | Type II | academic & research |
| 2019-08-15 | University of New Brunswick | Fredericton | NB | Type II | academic & research |
| 2019-08-15 | University of New Brunswick | Fredericton | NB | Type II | academic & research |
| 2019-08-15 | Fowler Construction Company Ltd. | Bracebridge | ON | Type II | industrial |
| 2019-08-15 | North Bay General Hospital | North Bay | ON | Type II | medical |
| 2019-08-15 | North Bay General Hospital | North Bay | ON | Type II | medical |
| 2019-08-15 | Trans Mountain Pipeline ULC | Kamloops | BC | Type II | industrial |
| 2019-08-15 | Acuren Inc. | Kamloops | BC | Type II | industrial |
| 2019-08-20 | Englobe Corp. | Baie Comeau | QC | Type II | industrial |
| 2019-08-20 | Englobe Corp. | Baie Comeau | QC | Type II | industrial |
| 2019-08-20 | SNC-Lavalin GEM Québec Inc. | Baie-Comeau | QC | Type II | industrial |
| 2019-08-20 | Centre intégré de santé et de services sociaux de la Côte-Nord | Baie-Comeau | QC | Type II | medical |
| 2019-08-21 | Alberta Power (2000) Ltd. | Hanna | AB | Type II | industrial |
| 2019-08-21 | Resolute FP Canada Inc. | Baie-Comeau | QC | Type II | industrial |
| 2019-08-21 | 8109796 Canada Inc. | Port-Cartier | QC | Type II | industrial |
| 2019-08-22 | Centre de santé et de services sociaux de Sept-Îles | Sept-Iles | QC | Type II | medical |
| 2019-08-22 | Englobe Corp. | Sept-Iles | QC | Type II | industrial |
| 2019-08-22 | Englobe Corp. | Sept-Iles | QC | Type II | industrial |
| 2019-08-22 | Centre intégré de santé et de services sociaux de la Côte-Nord | Baie-Comeau | QC | Type II | medical |
| 2019-08-22 | GHD Consultants Ltd. | Sept-Iles | QC | Type II | industrial |
| 2019-08-26 | Alamos Gold Inc. | Matachewan | ON | Type II | industrial |
| 2019-08-26 | Advanced Cyclotron Systems Inc. | Richmond | BC | Type II | commercial |
| 2019-08-26 | EXP Services Inc. | Timmins | ON | Type II | industrial |
| 2019-08-27 | Caron Equipment Inc. | Timmins | ON | Type II | industrial |
| 2019-08-28 | TBT Engineering Limited | Thunder Bay | ON | Type II | industrial |
| 2019-08-28 | Teranorth Construction & Engineering Limited | Cochrane | ON | Type II | industrial |
| 2019-08-28 | BCG Engineering Inc. | Kamloops | BC | Type II | industrial |
| 2019-08-28 | Detour Gold Corporation | Cochrane | ON | Type II | industrial |
| 2019-08-29 | C. Villeneuve Construction Co. Ltd. | Hearst | ON | Type II | industrial |
| 2019-08-29 | Morin Construction Ltd. | Hearst | ON | Type II | industrial |
| 2019-08-29 | EXP Services Inc. | Timmins | ON | Type II | industrial |
| 2019-08-30 | Saskatchewan Cancer Agency | Regina | SK | Type I | medical |
| 2019-09-01 | Acuren Inc. | Dartmouth | NS | Type II | industrial |
| 2019-09-04 | Nordion (Canada) Inc. | Laval | QC | Type II | industrial |
| 2019-09-04 | McGill University Health Centre | Montréal | QC | Type II | medical |
| 2019-09-04 | McGill University Health Centre | Montréal | QC | Type II | medical |
| 2019-09-04 | Mistras Services Inc. | Saint-Lambert | QC | Type II | industrial |
| 2019-09-08 | Xcisision Medical Systems, LLC | Ottawa | ON | Type II | commercial |
| 2019-09-09 | Canadian Blood Services | Dartmouth | NS | Type II | medical |
| 2019-09-09 | Welltec Canada Inc. | Bonnyville | AB | Type II | industrial |
| 2019-09-10 | IRISNDT Corp. | Cold Lake | AB | Type II | industrial |
| 2019-09-10 | Aecom Canada Ltd. | Calgary | AB | Type II | industrial |
| 2019-09-10 | Aecom Canada Ltd. | Calgary | AB | Type II | industrial |
| 2019-09-10 | Solidearth Geotechnical Inc. | Cold Lake | AB | Type II | industrial |
| 2019-09-10 | Wood Canada Limitée | Bonnyville | AB | Type II | industrial |
| 2019-09-10 | Atlantic Gold Corporation | Middle Musquodoboit | NS | Type II | industrial |
| 2019-09-11 | IMP Group Limited | Hammonds Plains | NS | Type II | commercial |
| 2019-09-11 | Tuboscope Vetco Canada ULC | Bonnyville | AB | Type II | industrial |
| 2019-09-11 | GHD Consultants Ltd. | Gatineau | QC | Type II | industrial |
| 2019-09-11 | Cenovus Energy Inc. | Bonnyville | AB | Type II | industrial |
| 2019-09-11 | Louisiana - Pacific Canada Ltd. | East River | NS | Type II | industrial |
| 2019-09-11 | Provincial Health Services Authority | Abbotsford | BC | Type II | medical |
| 2019-09-12 | Englobe Corp. | Havre-Saint-Pierre | QC | Type II | industrial |
| 2019-09-12 | Labatt Brewing Company Ltd. | Halifax | NS | Type II | industrial |
| 2019-09-12 | Natural Resources Canada | Dartmouth | NS | Type II | academic & research |
| 2019-09-12 | Fisheries and Oceans Canada | Dartmouth | NS | Type II | academic & research |
| 2019-09-13 | Provincial Health Services Authority | Surrey | BC | Type II | medical |
| 2019-09-16 | Allnorth Consultants Limited | Grande Prairie | AB | Type II | industrial |
| 2019-09-16 | Pump House Brewery Ltd. | Moncton | NB | Type II | industrial |
| 2019-09-16 | Anode NDT Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-09-17 | Université de Moncton | Moncton | NB | Type II | academic & research |
| 2019-09-17 | Régie régionale de la santé A | Moncton | NB | Type II | medical |
| 2019-09-17 | Régie régionale de la santé A (Zone 1A) | Moncton | NB | Type II | medical |
| 2019-09-17 | Queen Elizabeth II Hospital | Grande Prairie | AB | Type II | medical |
| 2019-09-17 | Queen Elizabeth II Hospital | Grande Prairie | AB | Type II | medical |
| 2019-09-17 | Intrepid NDE Testing Corp. | Grande Prairie | AB | Type II | industrial |
| 2019-09-17 | Superior General Partner Inc. | Grande Prairie | AB | Type II | industrial |
| 2019-09-17 | S.G.H. Inspection Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-09-17 | Anode NDT Ltd. | Grande Prairie | AB | Type II | industrial |
| 2019-09-18 | Diagnostic Imaging | Charlottetown | PE | Type II | medical |
| 2019-09-18 | Diagnostic Imaging | Charlottetown | PE | Type II | medical |
| 2019-09-18 | Gamma-Tech Inspection Ltd. | Calgary | AB | Type II | industrial |
| 2019-09-18 | 20/20 ND Technology Inc. | Grande Prairie | AB | Type II | industrial |
| 2019-09-18 | Stantec Consulting Ltd. | Charlottetown | PE | Type II | industrial |
| 2019-09-18 | Voltage Wireline Inc. | Grande Prairie | AB | Type II | industrial |
| 2019-09-18 | Fundy Engineering & Consulting Limited | Clyde River | PE | Type II | industrial |
| 2019-09-19 | PEI Department of Transportation, Infrastructure and Energy | Mount Stewart | PE | Type II | industrial |
| 2019-09-19 | PEI Department of Transportation, Infrastructure and Energy | Mount Stewart | PE | Type II | industrial |
| 2019-09-19 | Agnico Eagle Mines Limited | Nunavut | NT | Type II | industrial |
| 2019-09-19 | Englobe Corp. | Moncton | NB | Type II | industrial |
| 2019-09-19 | 20/20 ND Technology Inc. | Grande Prairie | AB | Type II | industrial |
| 2019-09-19 | Metalcare Group Inc. | Edmonton | AB | Type II | industrial |
| 2019-09-19 | International Paper Company | Grande Prairie | AB | Type II | industrial |
| 2019-09-20 | The Pepsi Bottling Group (Canada), ULC | Moncton | NB | Type II | industrial |
| 2019-09-23 | Weyerhaeuser Company Limited | Delta | BC | Type II | industrial |
| 2019-09-23 | Red Truck Beer Company Ltd. | Vancouver | BC | Type II | industrial |
| 2019-09-23 | High Precision Monitoring & Analysis Ltd. | Delta | BC | Type II | industrial |
| 2019-09-23 | Acuren Inc. | Regina | SK | Type II | industrial |
| 2019-09-23 | Acuren Inc. | Regina | SK | Type II | industrial |
| 2019-09-23 | WSP Canada Inc. | Edson | AB | Type II | industrial |
| 2019-09-23 | Mosaic Canada ULC | Belle Plaine | SK | Type II | industrial |
| 2019-09-24 | Vancouver Coastal Health Authority | Maple Ridge | BC | Type II | medical |
| 2019-09-24 | Millar Western Forest Products Ltd. | Whitecourt | AB | Type II | industrial |
| 2019-09-24 | ITL Testing Laboratories Ltd. | Maple Ridge | BC | Type II | industrial |
| 2019-09-24 | Buffalo Inspection Services (2005) Inc. | Swift Current | SK | Type II | industrial |
| 2019-09-24 | Reliance OFS Canada Ltd. | Whitecourt | AB | Type II | industrial |
| 2019-09-24 | Gunron Inspections Ltd. | Edson | AB | Type II | industrial |
| 2019-09-24 | Gibson Energy ULC | Moose Jaw | SK | Type II | industrial |
| 2019-09-25 | AM Inspection Limited | Cabri | SK | Type II | industrial |
| 2019-09-25 | North West Nuclear Medicine for Animals Inc. | Vancouver | BC | Type II | medical |
| 2019-09-25 | A-Tech N.D.T. Limited | Whitecourt | AB | Type II | industrial |
| 2019-09-25 | Slick Inspection Limited | Medicine Hat | AB | Type II | industrial |
| 2019-09-25 | TISI Canada Inc. | Regina | SK | Type II | industrial |
| 2019-09-25 | Trans Mountain Pipeline ULC | Surrey | BC | Type II | industrial |
| 2019-09-25 | WSP Canada Inc. | Swift Current | SK | Type II | industrial |
| 2019-09-25 | Pembina Pipeline Corporation | Whitecourt | AB | Type II | industrial |
| 2019-09-25 | Pembina Pipeline Corporation | Fox Creek | AB | Type II | industrial |
| 2019-09-26 | IRISNDT Corp. | Barrhead | AB | Type II | industrial |
| 2019-09-26 | AM Inspection Limited | Kindersley | SK | Type II | industrial |
| 2019-09-26 | AM Inspection Limited | Cabri | SK | Type II | industrial |
| 2019-09-26 | Advance Testing Ltd. | Surrey | BC | Type II | industrial |
| 2019-09-26 | KCS Plastics Ltd. | Langley | BC | Type II | industrial |
| 2019-09-26 | Slick Inspection Limited | Kindersley | SK | Type II | industrial |
| 2019-09-27 | Insight Medical Holdings Ltd. | Sherwood Park | AB | Type II | medical |
| 2019-09-27 | Miller Group Inc. | North Bay | ON | Type II | industrial |
| 2019-09-27 | B & B Contracting (2012) Ltd. | Surrey | BC | Type II | industrial |
| 2019-09-27 | Canadian Construction Materials Engineering & Testing Inc. | Surrey | BC | Type II | industrial |
| 2019-09-30 | Coco Paving Inc. | Oshawa | ON | Type II | industrial |
| 2019-10-01 | Orbit Engineering Limited | Brampton | ON | Type II | industrial |
| 2019-10-04 | Stanley Technical Services Ltd. | Olds | AB | Type II | industrial |
| 2019-10-04 | Winnipeg Regional Health Authority | Winnipeg | MB | Type I | commercial |
| 2019-10-07 | Structural Inspections Limited | Milton | ON | Type II | industrial |
| 2019-10-07 | 1629323 Alberta Ltd. | Drayton Valley | AB | Type II | industrial |
| 2019-10-07 | Mezei Inspections Ltd. | Drayton Valley | AB | Type II | industrial |
| 2019-10-08 | Royal Victoria Regional Health Centre | Barrie | ON | Type II | medical |
| 2019-10-08 | Canfor Pulp Ltd. | Prince George | BC | Type II | industrial |
| 2019-10-08 | Engtec Consulting Inc. | Vaughan | ON | Type II | industrial |
| 2019-10-08 | Prairie Mines & Royalty ULC | Edson | AB | Type II | industrial |
| 2019-10-08 | Prairie Mines & Royalty ULC | Edson | AB | Type II | industrial |
| 2019-10-08 | Four Corners Engineering Inc | Markham | ON | Type II | industrial |
| 2019-10-08 | Four Corners Engineering Inc | Markham | ON | Type II | industrial |
| 2019-10-09 | McMaster University | Hamilton | ON | Type II | academic & research |
| 2019-10-09 | Rio Tinto Fer et Titane inc. | Havre St-Pierre | QC | Type II | industrial |
| 2019-10-09 | V.A. Wood Associates Limited | Scarborough | ON | Type II | industrial |
| 2019-10-09 | V.A. Wood Associates Limited | Scarborough | ON | Type II | industrial |
| 2019-10-09 | Trans Mountain Pipeline ULC | Jasper | AB | Type II | industrial |
| 2019-10-09 | Trans Mountain Pipeline ULC | Jasper | AB | Type II | industrial |
| 2019-10-09 | Chinook Mine Construction Company Ltd. | Hinton | AB | Type II | industrial |
| 2019-10-09 | Chinook Mine Construction Company Ltd. | Hinton | AB | Type II | industrial |
| 2019-10-09 | Thompson Creek Metals Company Inc. | Prince George | BC | Type II | industrial |
| 2019-10-09 | Thompson Creek Metals Company Inc. | Prince George | BC | Type II | industrial |
| 2019-10-10 | University Health Network | Toronto | ON | Type II | medical |
| 2019-10-10 | University of Ontario Institute of Technology | Oshawa | ON | Type II | academic & research |
| 2019-10-10 | Milner Power Inc. | Grande Cache | AB | Type II | industrial |
| 2019-10-10 | Mackenzie Pulp Mill Corporation | Mackenzie | BC | Type II | industrial |
| 2019-10-10 | CST Canada Coal Limited | Grande Cache | AB | Type II | industrial |
| 2019-10-17 | Université de Moncton | Moncton | NB | Type II | academic & research |
| 2019-10-21 | NARL Refining | Come By Chance | NL | Type II | industrial |
| 2019-10-21 | NARL Refining | Come By Chance | NL | Type II | industrial |
| 2019-10-21 | Centre d'expertise en analyse environnementale du Québec | Québec | QC | Type II | academic & research |
| 2019-10-22 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Saint-Georges | QC | Type II | medical |
| 2019-10-22 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Saint-Georges | QC | Type II | medical |
| 2019-10-22 | Schlumberger Canada Limited | Mount Pearl | NL | Type II | industrial |
| 2019-10-22 | Texel Technical Materials | St-Elzéar-de-Beauce | QC | Type II | industrial |
| 2019-10-22 | Wolverine Mining Complex Limited | Tumbler Ridge | BC | Type II | industrial |
| 2019-10-22 | Wolverine Mining Complex Limited | Tumbler Ridge | BC | Type II | industrial |
| 2019-10-23 | Baker Hughes | Mount Pearl | NL | Type II | industrial |
| 2019-10-23 | Acuren Inc. | St. John's | NL | Type II | industrial |
| 2019-10-23 | Englobe Corp. | Thetford Mines | QC | Type II | industrial |
| 2019-10-23 | Canadian Forest Products Ltd. | Taylor | BC | Type II | industrial |
| 2019-10-23 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Thetford-Mines | QC | Type II | medical |
| 2019-10-23 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Thetford Mines | QC | Type II | medical |
| 2019-10-23 | Encana Corporation | Dawson Creek | BC | Type II | industrial |
| 2019-10-23 | Encana Corporation | Dawson Creek | BC | Type II | industrial |
| 2019-10-23 | Encana Corporation | Dawson Creek | BC | Type II | industrial |
| 2019-10-24 | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | commercial |
| 2019-10-24 | Les Entreprises Jacques Dufour & Fils Inc. | Baie-St-Paul | QC | Type II | industrial |
| 2019-10-24 | Jim Dent Construction Ltd. | Fort St John | BC | Type II | industrial |
| 2019-10-24 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Lévis | QC | Type II | medical |
| 2019-10-24 | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Lévis | QC | Type II | medical |
| 2019-10-24 | Louisiana - Pacific Canada Ltd. | Dawson Creek | BC | Type II | industrial |
| 2019-10-25 | Aker Solutions Asset Integrity and Management Canada Inc | St. John's | NL | Type II | industrial |
| 2019-10-25 | GHD Consultants Ltd. | St Catharines | ON | Type II | industrial |
| 2019-10-25 | MPI Moulin a Papier De Portneuf Inc. | Portneuf | QC | Type II | industrial |
| 2019-10-25 | Trenergy Inc. | St Catharines | ON | Type II | industrial |
| 2019-10-25 | Saskatchewan Cancer Agency | Saskatoon | SK | Type I | medical |
| 2019-10-25 | Saskatchewan Cancer Agency | Saskatoon | SK | Type II | medical |
| 2019-10-25 | University of Saskatchewan | Saskatoon | Sk | Type II | medical |
| 2019-10-28 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-10-28 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-10-29 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-10-29 | Alberta Health Services | Calgary | AB | Type II | medical |
| 2019-10-29 | Laboratoires d'Expertises de Rivière-du-Loup Inc. | Rivière-du-Loup | QC | Type II | industrial |
| 2019-10-29 | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rimouski | QC | Type II | medical |
| 2019-10-29 | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rimouski | QC | Type II | medical |
| 2019-10-29 | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | ON | Type II | commercial |
| 2019-10-30 | Mistras Canada, Inc. | Calgary | AB | Type II | industrial |
| 2019-10-30 | F.F. Soucy WB General Partner Ltd. | Rivière-du-Loup | QC | Type II | industrial |
| 2019-10-30 | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rivière-du-Loup | QC | Type II | medical |
| 2019-10-30 | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rivière-du-Loup | QC | Type II | medical |
| 2019-11-05 | Interior Testing Services Ltd. | Kelowna | BC | Type II | industrial |
| 2019-11-05 | Emil Anderson Construction Co. Ltd. | Kelowna | BC | Type II | industrial |
| 2019-11-05 | Emil Anderson Construction Co. Ltd. | Kelowna | BC | Type II | industrial |
| 2019-11-06 | Fletcher Paine Associates Ltd. | Vernon | BC | Type II | industrial |
| 2019-11-06 | Tetra Tech Canada Inc. | Vernon | BC | Type II | industrial |
| 2019-11-07 | Interior Health Authority | Penticton | BC | Type II | medical |
| 2019-11-07 | Interior Health Authority | Penticton | BC | Type II | medical |
| 2019-11-07 | Trillium Health Partners | Mississauga | ON | Type II | medical |
| 2019-11-07 | Atomic NDT Ltd. | Sylvan Lake | AB | Type II | industrial |
| 2019-11-07 | Q Test Inspection Ltd. | Sylvan Lake | AB | Type II | industrial |
| 2019-11-07 | Radioprotection Inc. | Burlington | ON | Type II | commercial |
| 2019-11-07 | Stantec Consulting Ltd. | Markham | ON | Type II | industrial |
| 2019-11-08 | Bunge Canada Holdings I ULC | Hamilton | ON | Type II | industrial |
| 2019-11-13 | Albarrie Canada Limited | Barrie | ON | Type II | industrial |
| 2019-11-13 | Titan Non-Destructive Examination Services Ltd. | Didsbury | AB | Type II | industrial |
| 2019-11-13 | Agrium Inc. | Carseland | AB | Type II | industrial |
| 2019-11-14 | Canadian Blood Services | Ottawa | ON | Type II | medical |
| 2019-11-15 | ARA Engineering Ltd. | Calgary | AB | Type II | industrial |
| 2019-11-15 | Interior Health Authority | Vernon | BC | Type II | medical |
| 2019-11-15 | Interior Health Authority | Vernon | BC | Type II | medical |
| 2019-11-15 | Arthon Industries Limited | Kelowna | BC | Type II | industrial |
| 2019-11-15 | Sola Engineering Inc. | Vaughan | ON | Type II | industrial |
| 2019-11-18 | Graymar Equipment (2008) Inc. | Hamilton | ON | Type II | industrial |
| 2019-11-18 | Provincial Health Services Authority | Kelowna | BC | Type II | medical |
| 2019-11-19 | Carmeuse Lime (Canada) Limited | Dundas | ON | Type II | industrial |
| 2019-11-19 | Delta Beverages Inc. | Woodbridge | ON | Type II | industrial |
| 2019-11-20 | Provincial Health Services Authority | Kelowna | BC | Type I | medical |
| 2019-11-20 | Amhil Enterprises | Mississauga | ON | Type II | industrial |
| 2019-11-20 | Amhil Enterprises | Brantford | ON | Type II | industrial |
| 2019-11-20 | CGC Acquisition Corporation | Red Deer County | AB | Type II | industrial |
| 2019-11-20 | Chirom International Consultants Inc. | Calgary | AB | Type II | industrial |
| 2019-11-21 | Gazzola Paving Limited | Toronto | ON | Type II | industrial |
| 2019-11-21 | Acuren Inc. | Cantley | QC | Type II | industrial |
| 2019-11-22 | Interior Health Authority | Kelowna | BC | Type I | medical |
| 2019-11-22 | Ruetgers Canada Inc. | Hamilton | ON | Type II | industrial |
| 2019-11-22 | 2259351 Ontario Inc. O/A Riverside Cardiology and Diagnosti | Toronto | ON | Type II | medical |
| 2019-11-25 | Pembroke MDF Inc. | Pembroke | ON | Type II | industrial |
| 2019-11-26 | Mississauga Metals and Alloys | Brantford | ON | Type II | commercial |
| 2019-12-03 | Dart Canada Inc. | Scarborough | ON | Type II | industrial |
| 2019-12-05 | Huntsville District Memorial Hospital | Huntsville | ON | Type II | medical |
| 2019-12-05 | Roxul Inc. | Milton | ON | Type II | industrial |
| 2019-12-06 | Kodiak Quality Control Ltd. | Oakville | ON | Type II | commercial |
| 2019-12-06 | GHD Consultants Ltd. | Various cities | QC, ON | Type I | industrial |
| 2019-12-11 | Q Test Inspection Ltd. | Sylvan Lake | AB | Type II | industrial |
| 2019-12-12 | Apotex Inc. | Richmond Hill | ON | Type II | industrial |
| 2019-12-13 | 1388020 Ontario Corp. | Toronto | ON | Type II | medical |
| 2019-12-16 | AR Geotechnical Engineering Ltd. | Medicine Hat | AB | Type II | industrial |
| 2019-12-16 | Shubh Engineering & Project Management Inc. | Etobicoke | ON | Type II | industrial |
| 2019-12-16 | Acuren Inc. | Edmonton | AB | Type II | commercial |
| 2019-12-16 | Acuren Inc. | Edmonton | AB | Type II | industrial |
| 2019-12-17 | Centre for Addiction and Mental Health | Toronto | ON | Type II | academic & research |
| 2019-12-17 | Centre for Addiction and Mental Health | Toronto | ON | Type II | medical |
| 2019-12-17 | Centre for Addiction and Mental Health | Toronto | ON | Type II | medical |
| 2019-12-17 | Express Pipeline Ltd. | Suffield | AB | Type II | industrial |
| 2019-12-17 | Trans Mountain Pipeline ULC | Stony Plain | AB | Type II | industrial |
| 2019-12-17 | Nova Scotia Health Authority | Sydney | NS | Type II | medical |
| 2019-12-17 | Step Energy Services Ltd. | Redcliff | AB | Type II | industrial |
| 2019-12-17 | Parkland Geotechnical Consulting Ltd. | Sherwood Park | AB | Type II | industrial |
| 2019-12-17 | Nova Scotia Health Authority | Sydney | NS | Type II | medical |
| 2019-12-18 | Misericordia Community Hospital | Edmonton | AB | Type II | medical |
| 2019-12-18 | Misericordia Community Hospital | Edmonton | AB | Type II | medical |
| 2019-12-18 | LAW Inspection Services Inc. | Dunmore | AB | Type II | industrial |
| 2019-12-18 | Slick Inspection Limited | Medicine Hat | AB | Type II | industrial |
| 2019-12-18 | Alpha Adroit Engineering Ltd. | Edmonton | AB | Type II | industrial |
| 2019-12-18 | High Precision Monitoring & Analysis Ltd. | Delta | BC | Type II | industrial |
| 2019-12-18 | Saint-Gobain Canada Inc. | Plattsville | ON | Type II | industrial |
| 2019-12-18 | WSP Canada Inc. | Markham | ON | Type II | industrial |
| 2019-12-19 | MPE Engineering Ltd. | Edmonton | AB | Type II | industrial |
| 2019-12-19 | Wood Canada Limitée | Medicine Hat | AB | Type II | industrial |
| 2019-12-19 | Frontop Engineering Limited | Markham | ON | Type II | industrial |
| 2019-12-19 | Porocel of Canada, Ltd. | Medicine Hat | AB | Type II | industrial |
| 2019-12-19 | ProtechGeo & Material Testing Ltd. | Edmonton | AB | Type II | industrial |
| 2019-12-19 | National Research Council of Canada | Edmonton | AB | Type II | academic & research |
| 2019-12-20 | Environment and Climate Change Canada | Edmonton | AB | Type II | academic & research |
Appendix G: Regulatory Program for Class IB Accelerator Facilities
| Facility | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| Inspections |
Licensing (person-days) |
Compliance (person-days) |
Inspections |
Licensing (person-days) |
Compliance (person-days) |
|
| TRIUMF | 1 | 6 | 115 | 2 | 4 | 133 |
| CLSI | 2 | 8 | 63 | 1 | 5 | 134 |
Appendix H: Safety and Control Rating for Class IB Accelerator Facilities
Table 21 and 22 present the rating for all 14 SCAs for TRIUMF and CLSI respectively from 2015 to 2019. Appendix M provides a description of the rating.
| Safety and control area | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Management system | SA | SA | SA | BE | BE |
| Human performance management | SA | SA | SA | SA | SA |
| Operating performance | SA | SA | SA | SA | SA |
| Safety analysis | SA | SA | SA | SA | SA |
| Physical design | SA | SA | SA | SA | SA |
| Fitness for service | SA | SA | SA | SA | SA |
| Radiation protection | FS | SA | SA | SA | SA |
| Conventional health and safety | SA | SA | SA | SA | SA |
| Environmental protection | SA | SA | SA | SA | SA |
| Emergency management and fire protection | SA | SA | SA | SA | SA |
| Waste management | SA | BE | SA | SA | SA |
| Security | SA | SA | SA | SA | SA |
| Safeguards and non-proliferation | FS | FS | FS | FS | FS |
| Packaging and transport | SA | SA | SA | SA | SA |
| Safety and control area | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Management system | SA | BE | SA | SA | BE |
| Human performance management | BE | SA | SA | SA | SA |
| Operating performance | SA | SA | SA | FS | FS |
| Safety analysis | SA | SA | SA | SA | SA |
| Physical design | FS | FS | FS | FS | FS |
| Fitness for service | SA | FS | FS | FS | FS |
| Radiation protection | FS | FS | FS | FS | FS |
| Conventional health and safety | SA | SA | FS | FS | FS |
| Environmental protection | SA | SA | FS | FS | FS |
| Emergency management and fire protection | SA | SA | SA | SA | SA |
| Waste management | FS | FS | FS | SA | SA |
| Security | FS | FS | FS | SA | SA |
| Safeguards and non-proliferation* | N/A | N/A | N/A | N/A | N/A |
| Packaging and transport | FS | FS | FS | FS | FS |
*N/A: CLSI does not have nuclear substances, equipment or information which need to be safeguarded. Therefore, CNSC staff do not perform any safeguards verification activities at CLSI.
Appendix I: Effective Doses to the Public from Class IB Accelerator Facilities
The 2018 and 2019 maximum effective doses to a member of the public due to airborne or liquid effluent releases of nuclear substances from TRIUMF are shown in Table 23. The main factor which caused variation of these values is the 520 MeV cyclotron annual delivered beam charge, which determines the total activity of short lived isotopes generated in the air adjacent to the main beamlines. During the last five years, the dose to a member of the public has remained at a level equivalent to about 1% of the CNSC regulatory dose limit of 1 mSV/year.
There are no airborne or liquid effluent releases of nuclear substances from CLSI. The environmental radiation levels outside the main CLSI building, as monitored by CLSI are at ambient background radiation levels. Therefore, the estimated dose to the public is at natural radiation background levels.
| Dose Data | 2013 | 2014 | 2015 | 2016 | 2017 | Regulatory Limit |
|---|---|---|---|---|---|---|
| Maximum effective dose (mSv) | 0.011 | 0.010 | 0.007 | 0.005 | 0.006 | 1 mSv/year |
Appendix J: Lost Time-Injury Rate for Class IB Accelerator Facilities
A key performance indicator for conventional health and safety SCA is the lost-time injury rate per 100 person-years. A lost-time injury is an injury or illness resulting in lost days beyond the date of the injury as a direct result of an occupational injury or illness incident. The ultimate target is to have a lost-time injury rate of zero.
Table 24 summarizes the lost-time injury rate by Class IB accelerator facilities from 2015 to 2019.
| Facility | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| TRIUMF | 0.9 (3) | 0.0 (0) | 0.5 (3) | 0.2 (1) | 0.6 (4) |
| CLSI | 0.4 (1) | 0.0 (0) | 0.4 (1) | 0.0 (0) | 0.0 (0) |
J.1 TRIUMF
In 2018, TRIUMF had one lost-time injury resulting in a lost-time injury rate of 0.15 per 100 person-years. In 2019, there was an increase to four lost-time injuries, resulting in a lost-time injury rate of 0.58 per 100 person-years. This is a factor of two more than TRIUMF target key performance indicator of 0.3 per 100 person-years.
Table 25 described the lost time injuries for the reporting period. None of the injuries was directly related to the licensed activities.
In response to this increase in 2019, TRIUMF updated its pre-job hazard analysis and briefing form for work involving contractors. TRIUMF will roll out this form site-wide for non-contractor work in 2020.
WorkSafeBC, the provincial agency with the mandate to oversee a non-fault insurance system for the workplace in British Columbia, has assigned TRIUMF in the advanced research classification unit along with similar businesses in British Columbia, such as institutions which provide post-secondary education including university, college, business, computer, career training, trade, and vocational programs. For both 2018 and 2019, the average lost-time injury rate for the advanced research classification unit was 0.6 for 100 person-years. Therefore, TRIUMF lost-time injury rate was well below the average rate for the advanced research classification unit in 2018, but was at the average rate for 2019.
J.2 CLSI
In 2018 and 2019, there was no lost-time injuries at CLSI.
| Injury | Lost-time (days) |
|---|---|
|
Hand laceration While a worker and a co-worker were working, a fly landed on the production table between them. Both of them went to swat the fly off the table. The co-worker was holding a scalpel and struck the worker right ring finger on the distal end. This resulted in the need for 6 stiches at the hospital and a tetanus shot. The worker did not come to work the next day. |
1 |
|
Slip and Fall A worker slipped in the snow and fell in the parking lot going to his car after work. The parking was ploughed, but not salted. The worker slipped backwards/sidewards onto his left arm and his head made slight contact with the ground. |
1 |
|
Debris in eye A worker positioned himself under the counter to unscrew a sink. He was not wearing any eye protection. As he started to unscrew the sink, some debris came loose and fell downwards into his right eye. He first rubbed his eye to remove the debris, then he walk to the eyewash station to use the eyewash bottles located about 6 feet away from where he was. He then felt better. The incident happened on a Friday of a long weekend. On Tuesday following the long weekend, the eye still had some irritation, so he went to a medical clinic for assessment, where he was referred to an optometrist. |
2 |
|
Back Strain A worker strained some muscles by pulling and sliding a heavy filled cinder block towards himself from an awkward position. |
3 |
|
Object fell onto hand A counter top fell and struck the hand of a worker, causing an injury to his finger, which has required surgery. |
25 |
Appendix K: Effective Doses to Workers at Class IB Accelerator Facilities
As shown in Figure 18, TRIUMF monitored the effective doses for 405 NEWs in 2018 and 433 NEWs in 2019. The maximum individual effective dose received by a NEW was during the third quarter of 2019 and was 9.18 mSv, or approximately 18 percent of the regulatory effective dose limit of 50 mSv in a one-year dosimetry period. This maximum individual effective dose was and unusual occurrence and is described in the Events section below.
TRIUMF also monitored the effective doses 907 non-NEWs in 2018 and 1091 non-NEWs in 2019, with a maximum effective dose of 0.18 mSv.
Effective doses at TRIUMF continue to be at the same level as previous years.
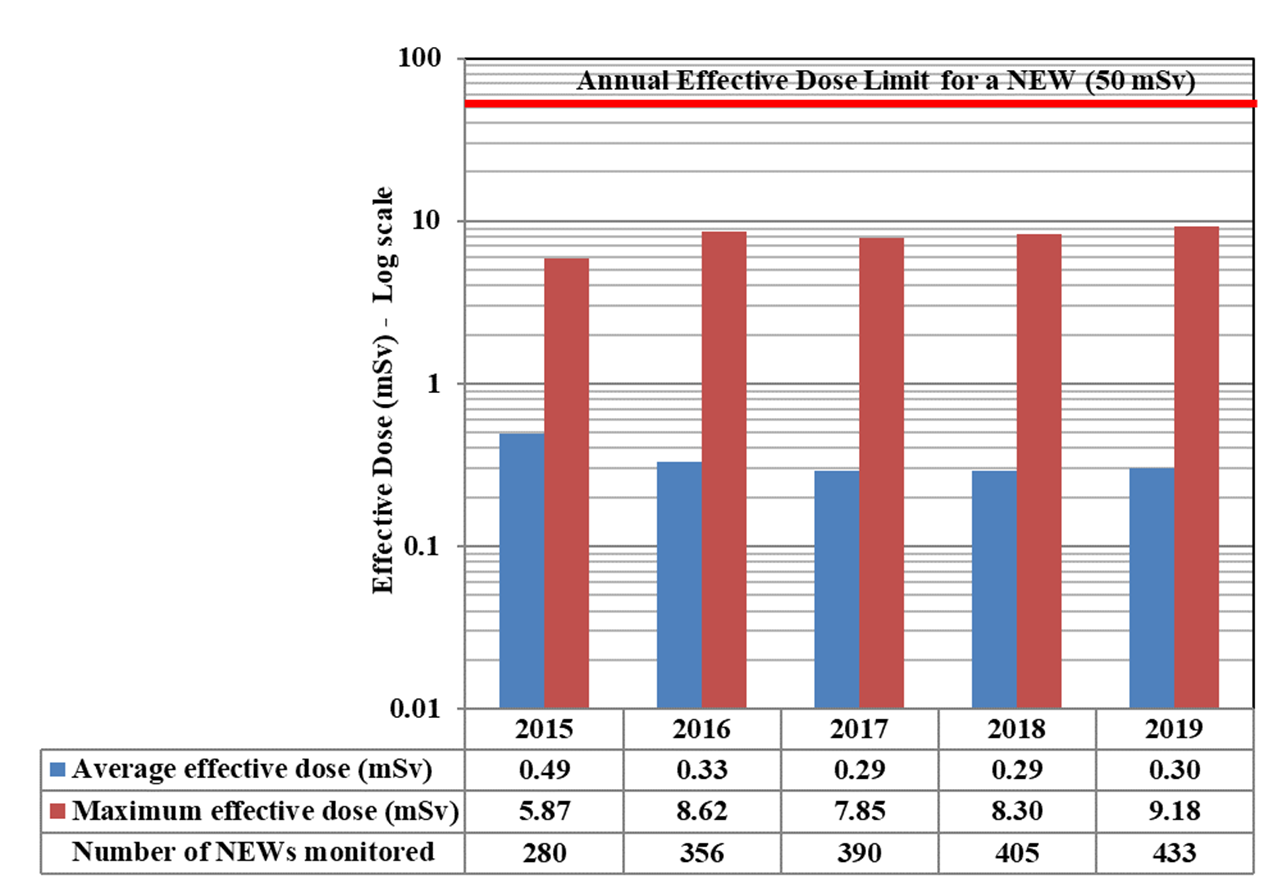
Figure 18: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Average effective dose (mSv) | 0.49 | 0.33 | 0.29 | 0.29 | 0.30 |
| Maximum effective dose (mSv) | 5.87 | 8.62 | 7.85 | 8.30 | 9.18 |
| Number of NEWs monitored | 280 | 356 | 390 | 405 | 433 |
| *Annual Effective Dose Limit for a NEW (50 mSv) | |||||
As shown in Figure 19, CLSI monitored the effective doses for 171 NEWs in 2018 and 129 NEWs in 2019. The maximum individual effective dose received by a NEW was 0.15 mSv, or 0.3 percent of the regulatory effective dose limit of 50 mSv in a one-year dosimetry period.
In 2018, CLSI also monitored the effective doses for 755 non-NEWs employees, users of the facility and contractors, with a maximum effective dose of 0.11 mSv. CLSI monitored 74 non-NEW employees, users and contractors with a maximum effective dose of 0.09 mSv in 2019. This reduction in the number of monitored non-NEWs employees, users of the facility and contractors by a factor of about 10 in 2019 is due to a CLS redefinition and restructuring of personnel dosimetry assignments in 2018. As a result of this change, non-NEW employees, users and contractors are not required to wear personal dosimetry in the controlled access areas of the facility. Dose for these groups of people are be ascertained using a network of passive area radiation monitors in different areas of the facility. In 2019, the passive area radiation monitors in these areas of the building did not record a value that would have resulted in any dose to these individuals and thus are assigned no dose.
Effective doses at CLSI continue to be low and are following the same trend as previous years.
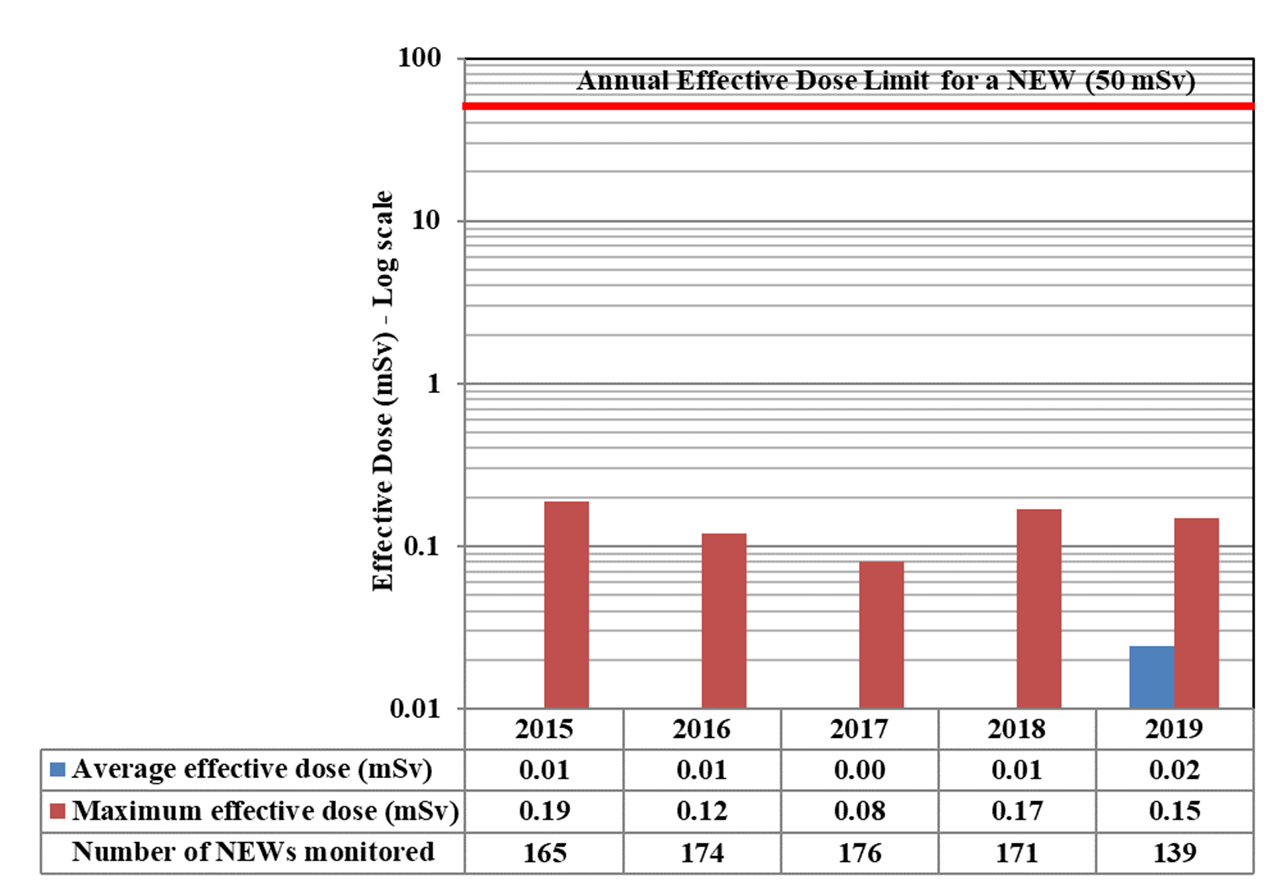
Figure 19: Text version
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Average effective dose (mSv) | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 |
| Maximum effective dose (mSv) | 0.19 | 0.12 | 0.08 | 0.17 | 0.15 |
| Number of NEWs monitored | 165 | 174 | 176 | 171 | 139 |
| *Annual Effective Dose Limit for a NEW (50 mSv) | |||||
Appendix L: Events at Class IB Accelerator Facilities
L.1 TRIUMF
January 2018: Two contaminated TRIUMF F-308 shipping containers at the Canadian Nuclear Laboratories (TRIUMF non-compliance report NCR#10969)
Upon unloading at Canadian Nuclear Laboratories (CNL, Chalk River, Ontario), it was discovered that two of five F-308 shipping flasks (Type A packages) containing spent targets form the ISAC facility had been contaminated on route after leaving TRIUMF on January 5, 2018. The contamination was restricted to the flask and no one was contaminated. Following decontamination of the external surfaces of the flasks by CNL personnel, they were returned to TRIUMF where further detailed analysis of the internal surfaces strongly suggested that the bottom of one of the 22.7 litre steel inner pails had been breached during transport. The suspected cause of the breach was mechanical fatigue induced by stresses from movement of the target inside the pail during transport.
The principal corrective action was to source a heavier-gauge steel pail that would be more resistant to the stresses. A controlled "simulated road test" was performed to assess the validity of both the presumed cause and the proposed corrective action by reproducing the vibrational stresses resulting from several thousand kilometers of road travel. The details and results of this test confirmed both the assumed cause and the effectiveness of the proposed solution.
February 15, 2018: Powdered target lithium hydride fire (TRIUMF non-compliance report NCR#10871)
In 2018 a site-wide 5S initiative was undertaken to clean-up and remove surplus equipment and detectors that had accumulated from the last several decades of operation. 5S is a workplace organization method that stands for sort, set in order, shine, standardize, and sustain. During a focused 5S event in the Meson Flail, an unlabeled 1-litre size metal target canister, containing several hundred grams of reactive material (likely lithium hydride) was removed from the Meson Hall and placed in the metal recycling area on the south side. While there, the target window was accidentally punctured when a tool fell on it, dispersing a thin layer of powder on the supporting block and asphalt floor. Not realizing that it was a reactive metal, the worker placed the metal canister upright in an outdoor waste bin where it was exposed to moisture and started smoldering. Prompt action on the worker’s part in summoning assistance resulted in the fire being quickly extinguished. Vancouver Fire & Rescue Services responded with their hazardous materials team.
A root cause analysis was completed that recommended a corrective action to document a safe work procedure for conducting a 5S event. The procedure includes steps for planning and conducting a 5S event that ensure hazards are assessed ahead of time and that safety personnel input is solicited in deciding on safe disposal of surplus material.
October 9, 2018: Accidental release of carbon-11
On October 9, 2018, there was an unplanned release of ~33 GBq of gaseous C-11, which occurred during the production of PET pharmaceuticals in a Life Sciences Division radioisotope library. The cause of the release was determined to have been “operator error” on the part of the chemist. Based on the wind direction over the time of the release, it was estimated that the maximum dose to a member of the public as a result of the lease would have been approximately 8.5 nSv. The impact to member of the general public corresponded to less than 1/100,000 of the regulatory limit. Changes were made to the procedure to minimize the likelihood of recurrence of such an error.
October 19, 2018: 12.5 kV power line strike (TRIUMF non-compliance report NCR#11750)
On October 19, 2018 a sub-contractor retained by WSP Environmental to complete environmental sampling work for the Institute for advanced Medical Isotopes (IAMI) project struck the buried 12.5 kV high voltage line that supplies power to the TRIUMF site. The workers were not injured. Owing to redundancies in the number of cables in the duct bank, connections were able to be reconfigured to use the undamaged cables, and power to the site was re-established within 30 hours. A full ramp up of machine operation took another three days. An investigation was done and found that the locating service contractor retained by WSP Environmental did not locate the concrete duct bank that runs between the substation immediately south of the perimeter fence to the site transformers. The investigation also revealed that the WSP engineer had not reviewed site services drawings even when making changes to hole locations late in the process, and they had not attended the site orientation session held earlier for IAMI contractors. The incident was immediately reported to regulatory authorities, WorkSafeBC, Technical Safety BC and the CNSC. TRIUMF put a moratorium on all ground disturbances until a review could be completed and new controls put in place.
The root causes identified the lack of formal TRIUMF procedures and controls for ground disturbance activities on site. This has been rectified with the implementation of a new Ground Disturbance Permit and associated Work Instruction: Ground Disturbance Procedure. The new proceduress require: use of up to date site utility drawings obtained from TRIUMF Service Group engineers; overlay of the proposed ground disturbances on the utility drawings; use of a contractor for locating underground utilities; and a final review by TRIUMF services engineers of the proposed ground disturbances with the located utilities to determine if further non-destructive techniques are required to expose existing utilities prior to excavation.
January 8, 2019 and October 28, 2019: Accidental Releases of carbon-11 (TRIUMF non-compliance report NCR#12818)
Two accidental releases of C-11 from Lab 005 during post-irradiation processing occurred, the first on January 8 (52 GBq) and the second on October 28 (15 GBq). Based on analyses of the release profiles that take into account the wind direction over the duration of the releases, the first release is estimated to have a maximum dose impact to a member of the public of 16 nSv, the second 3 nSv. Their combined impact to member of the general public corresponded to less than 1/50,000 of the regulatory limit. The cause of the first release was determined to be that the re-assembly of the apparatus following maintenance had not been sufficiently tight to prevent leakage from the trapping column. An additional step was added to the processing checklist to ensure that the apparatus was leak tight prior to commencing the process.
The cause of the second release was operator error: the chemist performing the process pushed the wrong btn on the control console, causing the activity to be released into the nuclear ventilation stack instead of the waste bag. The corrective actions included making hardware changes that would prevent the release of activity directly into the nuclear ventilation, as well as making changes to the colours and locations of control btns to reduce the likelihood of the same error recurring.
January 24 to March 27, 2019: Accidental releases of xenon-123 and iodine-123 (TRIUMF non-compliance report NCR#12368)
A leaky BWXT gas target resulted in the release of 98 GBq of Xe-123 and 170 MBq of I-123 during irradiations over a period of approximately two months. An additional release of 11 GBq of Xe-123 occurred during June 17-18 when the same target was used again following an extended maintenance period for the CP42. The releases were identified from observations of I-123 on the weekly nuclear exhaust stack sampling filters. The Xe-123 releases were subsequently identified in the logged air monitor data from their distinctive build-up curves. Corrective actions included training for RPG surveyors to recognize and report anomalous occurrences, as well as providing ATG Operators with information to allow them to identify and report anomalous air monitor readings that may indicate leaking targets but are below alarm threshold. Based on an analysis of the release profile that takes into account the wind direction over the duration of the releases, the releases are estimated to have a maximum dose impact to a member of the public of 21 nSv. The impact to member of the general public corresponded to less than 1/30,000 of the regulatory limit.
May 28, 2019 and August 27. 2019: Accidental release of krypton-79, krypton-77 and bromine-77 (TRIUMF non-compliance reports NCR#12295 and NCR#12613)
Although the original release in May (40 GBq Kr-79) was attributed to a leaky valve stem on the rubidium target canister, the proposed cause was never verified. Following the second release (194 GBq Kr-79) the original assumptions were re-evaluated in light of evidence of overheating of the target canisters, which included bulging end windows and discoloration marks on the target barrels. The root cause was determined to be overheating resulting from reduced cooling water flow to the windows, which put too much mechanical stress on the windows. The flow reduction was in turn determined to have resulted from changes made to the cooling system during the previous winter shutdown. Based on analyses of the release profiles that take into account the wind direction over the duration of the releases, the first release is estimated to have a maximum dose impact to a member of the public of 60 nSv, the second 11 nSv. Their combined impact to member of the general public corresponded to less than 1/12,000 of the regulatory limit.
Corrective actions included:
- Updates to the expected air monitor alarm response for the main control room operators
- Installation of air monitor read-outs and alarm annunciation at the BL2C STF hot cell
- Adoption of a requirement to have a formal decision-making process for authorizing the resumption of irradiations following a target failure
- Adoption of a requirement to review any proposed changes to the system configuration prior to adoption, in particular any potential safety-related implications
- Consideration of changes to the target and target holder design to make it more robust against the effects of overheating.
December 12, 2019: Improperly sealed Type A container; damaged Type A container (TRIUMF non-compliance report NCR#12933)
This incident actually comprises two separate dangerous occurrences per the Packaging and Transport of Nuclear Substances Regulations. In the first incident the lock ring that secures the lid to the outer container of a (borrowed) Type A container, came open from casual contact in the Toronto Airport Air Canada freight handling facility. The container has a mechanism to prevent such occurrences but it was not used. The subsequent investigation determined that this was due to incomplete knowledge of the proper use of the container by the people who packed it. The second incident occurred after the lid had been replaced and secured in the intended manner, when the container fell while being loaded onto the plane, suffering irreparable damage. There were no radiation exposure to the worker or to the public and there were no releases to the environment resulting from these two incidents.
Corrective actions included revision of TRIUMF’s shipping protocols to prevent the use of any Type A containers for which TRIUMF does not possess proof of the package’s certification for its intended use, and instructions for using it correctly. Additionally, improvements were specified to training for people involved in making radioactive shipments to ensure that everyone understands the requirements and has the necessary training credentials to allow them to perform such work.
January 20, 2020: Unexpectedly high quarterly dose for a TRIUMF NEW for third quarter of 2019 (TRIUMF non-compliance report NCR#13001)
The Q3, 2019 Landauer badge reading for a technician working in the ISAC experimental hall indicated a dose of 9.2
mSv had been incurred in Q3, 2019. Only one plausible source was suggested that could have led to the dose, exposure
to x-rays while working underneath electrostatic dipoles at the DRAGON facility when the high voltage was turned on.
The root cause investigation findings were that the facility Safety Analysis Report (SAR) indicated neither
engineered nor administrative controls that would have
mitigated this hazard under the circumstances pertaining at the time of the incident. Corrective actions included a
requirement to have a physical barrier in place to prevent access to the space immediately underneath the chambers
any time they are at high voltage, and that this requirement be reflected in the DRAGON Facility SAR. These
requirements also apply to EMMA which utilizes similar electrostatic dipoles.
Conclusion
TRIUMF staff filled internal non-compliance reports for each of the events above and implemented corrective and preventive actions to prevent reoccurrence. The six accidental releases of radioisotopes, the two transport container incidents, the fire incident and the 12.5 kV power line strike were immediately reported to CNSC as required by the regulation. TRIUMF also reported the latter two incidents to WorkSafeBC. None of the incidents resulted in injuries. The six accidental releases of radioisotopes resulted in an estimated maximum dose impact to the public of 120 nSv (1/8,000 of the regulatory limit), taking into account the wind direction over the duration of the releases. The high quarterly dose for a NEW for the third quarter of 2019 was below TRIUMF action level and therefore, TRIUMF did not immediately report this event to CNSC. TRIUMF provided information and corrective actions surrounding this event within the 2019 ACR as required.
L.2 CLSI
March 15, 2018: Access to the Free Access Zone by former user
A former user was able to gain facility access into the Free Access Zone. The person was identified promptly and escorted from the facility. Improvements to facility access process have been made to prevent the risk of a recurrence of this type of event.
June 24, 2018: Failure of electron gun
The high voltage power supply for the electron gun failed. Repairs were undertaken but the issue was not able to be resolved. Further investigation revealed significant damage occurred to several of the gun components and resulted in the machine being down for a 6- month period (July to December). The repairs were completed by mid-December and gun operation was restored on December 20.
February 17, 2019: Leak of 30% ethylene glycol in sewer system
An isolation joint for pump WP0105.1-22 ruptured leaking ~5,000 liters of 30% ethylene glycol in the sewer system. The pump was shut down, the isolation joint was repaired and glycol/water mixture was replaced into the system. The spill was considered a reportable event by the CNSC. The spill was also reported to the University of Saskatchewan, City of Saskatoon, Saskatchewan Spill Report Centre, and Environment Canada. CNSC Staff was satisfied with CLSI corrective actions to prevent reoccurrence. Such releases are diluted in the sewer system and are treated by the municipal water treatment plant prior to discharge. Therefore, the risk to the environment is minimal.
May 1, 2019: Faulty active area radiation monitoring system
The Active Area Radiation Monitoring System (AARMS) alarmed and interlocked at an incorrect value of 1.8 μSv cumulative dose on AARM1502-01. The AARMS should alarm at 25μSv as per design parameters for that location. The issue was found to be a factor of the AARMS Verification & Validation (V&V) testing that occurred a week earlier during preparations for accelerator startup. During the V&V process, inspectors change the values of the programmable logic controller (PLC) to simulate alarm conditions. The setpoint was properly set in the Input/Output controller but that setting was not replicated in the PLC. A provision to have the Controls Engineer verify the continuity of setpoints after a V&V has been completed and is now in place.
June 7, 2019: Security related event.
Prescribed information withheld from publication.
June 21, 2019: Beam on without the full minimum complement
Due to a scheduling error a Floor Coordinator did not arrive by the 8:00 am scheduled start time for the shift. The outgoing Floor Coordinator contacted HSE On-Call and was told to contact the Operator Lead, who supervises the operator group. The Supervisor did not answer so a message was left regarding the situation. At approximately 09:30 the Supervisor retrieved the message and made arrangements for the On-Call Floor Coordinator to fill in for the remainder of the shift. The On-Call Floor Coordinator arrived at 10:17 am. The remainder of the shift ran without incident. No safety concerns were noted as a result of the incident. Operators were trained to be clear that in the event the full minimum complement is not on –site, the beam must be turned off.
Conclusion
CLSI staff reported each of these incidents using the licensee’s internal reporting mechanism. Corrective and preventative actions were taken to prevent reoccurrence.
Appendix M: Compliance Rating Level
The following rating levels, as shown in table 26, reflect the transition in rating terminology used by the CNSC. While inspection reports may still use the previous rating levels, licensees that use nuclear substances and radiation devices can expect this transition to take place in time.
| Previous rating level | Description | New rating level | Description |
|---|---|---|---|
| A | Exceeds expectations | FS | Fully satisfactory |
| B | Meets expectations | SA | Satisfactory |
| C | Improvement is required | BE | Below expectations |
| D | This area is seriously compromised | ||
| E | Breakdown | UA | Unacceptable |
Fully satisfactory (FS)
Safety and control measures implemented by the licensee are highly effective. In addition, compliance with regulatory requirements is fully satisfactory, and compliance within the SCA exceeds requirements and CNSC expectations. Overall, compliance is stable or improving, and any problems or issues that arise are promptly addressed.
Satisfactory (SA)
Safety and control measures implemented by the licensee are sufficiently effective. In addition, compliance with regulatory requirements is satisfactory. Compliance within the SCA meets requirements and CNSC expectations. Any deviation is minor and any issues are considered to pose a low risk to the achievement of regulatory objectives and CNSC expectations. Appropriate improvements are planned.
Below expectations (BE)
Safety and control measures implemented by the licensee are marginally ineffective. In addition, compliance with regulatory requirements falls below expectations. Compliance within the SCA deviates from requirements or CNSC expectations to the extent that there is a moderate risk of ultimate failure to comply. Improvements are required to address identified weaknesses. The licensee is taking appropriate corrective action.
Unacceptable (UA)
Safety and control measures implemented by the licensee are significantly ineffective. In addition, compliance with regulatory requirements is unacceptable and is seriously compromised. Compliance within the SCA is significantly below requirements or CNSC expectations, or there is evidence of overall non-compliance. Without corrective action, there is a high probability that the deficiencies will lead to unreasonable risk. Issues are not being addressed effectively, no appropriate corrective measures have been taken and no alternative plan of action has been provided. Immediate action is required.
Appendix N: Regulatory Documents
N.1 Documents Under Development
- Amendments to the Radiation Protection Regulations (RPR) are currently ongoing to align with ICRP Publication 103 and IAEA’s Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards GSR Part 3 (2014). Application guide
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment is currently in development.
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment
- REGDOC-2.5.6, Design of Rooms Where Unsealed Nuclear Substances are Used
- REGDOC-2.6.2, Maintenance Programs for Nuclear Power Plants
- REGDOC-2.7.1, Radiation Protection
- REGDOC-2.7.2, Dosimetry, Volume I: Ascertaining Occupational Dose
- REGDOC-2.9.2, Environmental Protection: Controlling Releases to the Environment
- REGDOC-3.1.3, Reporting Requirements for Waste Nuclear Substance Licensees, Class II Nuclear Facilities and Users of Prescribed Equipment, Nuclear Substances and Radiation Devices Published in March 2020
- CSA PCP-09 Certified Exposure Device Operator Personnel Certification Guide
N.2 Documents Published in 2019
- Role of the Radiation Safety Officer: Final Evaluation ReportRegulatory focus in 2019. Published in September 2019.
- REGDOC-2.8.1, Conventional Health and Safety Published July 2019
- REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources and Category I, II and III Nuclear Material, Version 2 Published June 2019
Appendix O: Relevant Regulatory References
The following is a list of regulatory references that apply to the use of nuclear substances and prescribed equipment. The list is not exhaustive.
O.1 Act and regulations
- Nuclear Safety and Control Act
- Administrative Monetary Penalties Regulations (Canadian Nuclear Safety Commission)
- Class II Nuclear Facilities and Prescribed Equipment Regulations
- General Nuclear Safety and Control Regulations
- Nuclear Substances and Radiation Devices Regulations
- Packaging and Transport of Nuclear Substances Regulations, 2015
- Radiation Protection Regulations
- Transportation of Dangerous Goods Regulations
O.2 Regulatory documents
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment (draft)
- REGDOC-1.5.1, Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment
- REGDOC-1.6.1, Licence Application Guide: Nuclear Substances and Radiation Devices, Version 2
- REGDOC-2.2.3, Personnel Certification: Radiation Safety Officers
- REGDOC-2.2.3, Personnel Certification: Exposure Device Operators
- G-129, rev. 1, Keeping Radiation Exposures and Doses “As Low as Reasonably Achievable (ALARA)”
- REGDOC-2.9.1, Environmental Protection: Environmental Principles, Assessments and Protection Measures, Version 1.1
- REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources
- REGDOC-2.14.1, Information Incorporated by Reference in Canada’s Packaging and Transport of Nuclear Substances Regulations, 2015
- REGDOC-3.6, Glossary of CNSC Terminology
- Regulatory Policy P-290, Managing Radioactive Waste
Appendix P: Categorization of Sealed Sources
The category of sealed sources is a risk-based ranking developed by the International Atomic Energy Agency and described in IAEA, Categorization of Radioactive Sources, Safety Guide RS-G-1.9 (2005).
| Sealed Source Category | Risk | Description | Examples of usage |
|---|---|---|---|
| 1 | Very high risk | These sealed sources, if not safely managed or securely protected, would be likely to cause permanent injury (in some cases fatal) to a person handling or coming in contact with them for a period of a few minutes. Exposure would be fatal if a person were close to it in an unshielded manner for a few minutes to an hour |
|
| 2 | High risk | These sealed sources, if not safely managed or securely protected, could cause permanent injury to a person handling it, or coming in contact with them, for a short period of time (minutes to hours), or be fatal if close to it in an unshielded manner for a few days. |
|
| 3 | Moderate risk | These sealed sources, if not safely managed or securely protected, could cause permanent injury to a person either handling them, or, otherwise coming in contact with them, for some hours. Although unlikely, it could be fatal to be close to this amount of unshielded radioactive nuclear substances for a period of days to weeks. |
|
| 4 | Low risk | It is very unlikely that anyone would be permanently injured by these sealed sources. However, if this unshielded radioactive nuclear substance is not safely managed or securely protected, although unlikely, it could temporarily injure someone handling it, in contact with it, or who is close to it for several weeks. |
|
| 5 | Very low risk | No one could be permanently injured by this radioactive nuclear substance. |
|
Footnotes
- Footnote 1
-
The term "event" is defined in REGDOC-3.6, Glossary of CNSC Terminology
- Footnote 2
-
The word "event" is defined in REGDOC-3.6, Glossary of CNSC Terminology
Page details
- Date modified: